Contexts, ideas and activities to help you teach students the orbital model of the atom and bonding
Structure and bonding is about explaining the behaviour, reactions, properties and uses of macroscopic substances using models of matter at a sub-microscopic level. This topic plays a very large part in solving the world’s problems – not least in finding a vaccine for Covid-19, where molecular shape is important. The electrostatic interactions and intermolecular bonds determine the tertiary structure of many biological molecules, such as DNA, proteins (and therefore enzymes), hormones, skin, bone and muscle. Being able to understand how biological molecules work and what prevents them from working is essential if we want to optimise health and well-being.
Properties of materials are determined by their bonding and structure. This is clearly illustrated by considering different allotropes of carbon, where applications range from diamond in drill tips and graphite in pencil leads to carbon nanotubes for structural reinforcement in construction and for drug delivery to cells in pharmacy – just to name a few!
What students need to know
To understand structure and bonding, students need to know about the orbital model of the atom, plus covalent, ionic and metallic bonding:
- In an atom the positive nucleus is surrounded by constantly moving electrons which occupy different energy levels containing one or more sub-levels. Electronic structure influences the chemical properties of an element.
- Molecules are groups of atoms held together by intramolecular forces, such as covalent bonds. Weaker intermolecular forces exist between molecules. Valence Shell Electron Pair Repulsion (VSEPR) can be used to work out the shape of molecules and molecular ions. Macromolecular structures are formed from allotropes of carbon and silicon dioxide.
- Ionic bonding arises from the strong electrostatic attraction between ions of opposite charge, leading to giant ionic lattice structures. Metals have giant structures with positive ions in a lattice surrounded by a sea of delocalised electrons.
Threshold concept
The physical nature of bonding is key to understanding the topic. It’s all about electrostatics, built on the idea that opposites attract. There are attractions both within and between atoms and molecules. The electronegativity of an atom is how strongly it attracts electrons towards itself, which leads to polar molecules and intermolecular forces. Electrostatics need to be considered alongside energetics.
Misconceptions
Some common misconceptions your post-16 students may have include:
- Atoms need to gain full outer shells of electrons in order to be stable.
- Bonds involve the sharing or transfer of electrons.
- Bonding is either covalent or ionic.
- Ionic bonds occur when one atom transfers/gains one or more electrons to/from another atom.
- The number of electrons that an atom loses or gains determines the number of ionic bonds that that atom can form (eg, sodium can form one ionic bond with chlorine).
- A covalent bond occurs when two atoms share a pair of electrons between them.
You can read more about these, the research behind them and approaches to avoid them in the RSC’s Chemical misconceptions resources chemical structure and chemical bonding.
You can read more about these, the research behind them and approaches to avoid them in the RSC’s Chemical misconceptions resources chemical structure and chemical bonding: LINK; LINK.
Ideas for your teaching
Students often struggle with the abstract nature of structure and bonding. Teaching can be very dry as it can be difficult to find engaging activities. These ideas will help to get you started.
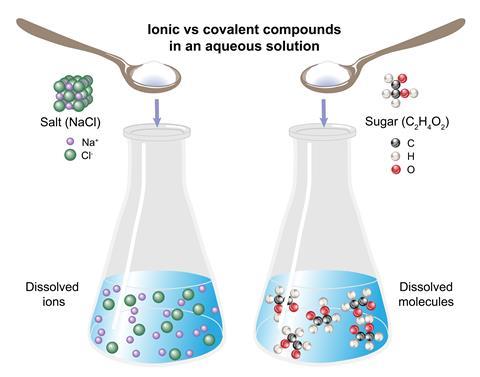
Use short, simple experiments with open-ended questions to get your students thinking about interactions between different substances. For example, put a blob of hair gel onto two petri dish lids. Gently sprinkle sodium chloride over the first blob and sugar over the second. Ask your students to explain why a clear liquid comes out of the hair gel when sodium chloride is added but not when sugar is added. Or put a spatula of sodium chloride into a beaker of water and stir. Repeat using ethanol instead of water. Again ask your students to explain why the salt is soluble in water but not in ethanol; after all, both solvents are covalently bonded.
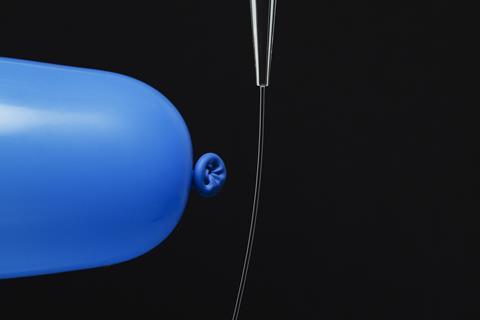
The balloons and static electricity simulation from PhET provides a good starting point for discussions focused around electrostatic attraction. Follow this up with an investigation into what happens when different charged plastics are held near a jet of water and a non-polar hydrocarbon. Ask your students to explain their observations. Use the RSC’s Jets of liquid resources for this activity. You can then go on to demonstrate the density of ice, which will encourage discussion around the existence of the hydrogen bond.
The reaction between zinc and iodine reveals that zinc iodide displays both ionic characteristics (ie electrolysis occurs in solution) and covalent characteristics (as it is dissolved in alcohol and ether). You can use this reaction to help students to appreciate that most compounds are not purely ionic or purely covalent – an idea that often arises from over-reliance on the octet rule.
To develop an understanding of covalent bonding in terms of energetic stability rather than full shells, you can give students the covalent bonding worksheet and discussion questions from the Chemistry for the gifted and talented series. You can then demonstrate the making of silicon and silanes from sand to explore the compounds of silicon before a discussion about group 4 elements. Ask your students why silicon dioxide is a solid with a giant structure while carbon dioxide is molecular, or why silanes react spontaneously with air at room temperature but alkanes are stable. These differences can be explained by considering the relevant bond energies and availability of d-orbitals in silicon but not in carbon.
Use short, simple experiments with open-ended questions to get your students thinking about interactions between different substances. For example, put a blob of hair gel onto two petri dish lids (LINK). Gently sprinkle sodium chloride over the first blob and sugar over the second. Ask your students to explain why a ’clear liquid’ comes out of the hair gel when sodium chloride is added but not when sugar is added. Or put a spatula of sodium chloride into a beaker of water and stir. Repeat using ethanol instead of water. Again ask your students to explain why the salt is soluble in water but not in ethanol; after all, both solvents are covalently bonded.
The balloons and static electricity simulation from PhET provides a good starting point for discussions focussed around electrostatic attraction: LINK. Follow this up with an investigation into what happens when different charged plastics are held near a jet of water and a non-polar hydrocarbon. Ask your students to explain their observations. Use the RSC’s Jets of liquid resources for this activity: LINK. You can then go on to demonstrate the density of ice, which will encourage discussion around the existence of the hydrogen bond.
The reaction between zinc and iodine reveals that zinc iodide displays both ionic characteristics (ie electrolysis occurs in solution) and covalent characteristics (as it is dissolved in alcohol and ether): LINK. You can use this reaction to help students to appreciate that most compounds are purely iconic or purely covalent – an idea that often arises from over reliance on the octet rule.
To develop an understanding of covalent bonding in terms of energetic stability rather than full shells, you can give students the covalent bonding worksheet and discussion questions from the Chemistry for the gifted and talented series: LINK. You can then demonstrate the making of silicon and silanes from sand to explore the compounds of silicon before a discussion about group 4 elements: LINK. Ask your students why silicon dioxide is a solid with a giant structure while carbon dioxide is molecular, or why silanes react spontaneously with air at room temperature but alkanes are stable. These differences can be explained by considering the relevant bond energies and availability of d-orbitals in silicon but not in carbon.
Assessment
The Chemical misconceptions resources offer some useful diagnostic probes to assess understanding, reveal misconceptions and promote discussion to help you discover what your students really think. These worksheet-based activities cover an analogy for the atom, chemical stability, why hydrogen and fluorine react, identifying bonds and interactions. You can use these at various points throughout this topic.
You can also use the series of short quizzes and activities from Starters for ten: atomic structure and bonding. Try them as retrieval practice at the start of a lesson or as an assessment activity after teaching the topic.
The Chemical misconceptions resources offer some useful diagnostic probes to assess understanding, reveal misconceptions and promote discussion to help you discover what your students really think. These worksheet-based activities cover an analogy for the atom (LINK), chemical stability (LINK), why hydrogen and fluorine react (LINK), identifying bonds (LINK) and interactions (LINK). You can use these at various points throughout this topic.
You can also use the series of short quizzes and activities from Starters for ten: atomic structure (LINK) and bonding (LINK). Try them as retrieval practice at the start of a lesson or as an assessment activity after teaching the topic.
Take-home points
- Try to introduce all forms of bonding as electrostatic phenomena.
- Don’t necessarily follow the order given in the specification as it doesn’t always build up conceptual understanding.
- Tell students the octet rule is a useful rule of thumb or memory aid for working out ion charges and formulas of compounds, but that it should never be used as an explanation. Atoms do not desire a full outer shell. Processes are driven by energetics.
- Be careful with the language you use to describe bonds as this can lead to confusion further down the line.
- Use simple practical activities to promote thinking.
- Use diagnostic probes to promote discussion and find out what your students think.
Dorothy Warren is an independent science education consultant based in the UK














1 Reader's comment