Try this class practical to investigate how temperature affects yeast and the expansion of bread dough
Yeast is a microbe used in bread making which feeds on sugar. Enzymes in yeast ferment sugar forming carbon dioxide and ethanol. The carbon dioxide makes the bread rise, while the ethanol evaporates when the bread is baked. In this experiment, students investigate the effect of different temperatures on yeast activity and the expansion of the bread dough.
It is best if each group does the activity at one temperature and then shares the results with other groups.
Equipment
Apparatus
- Spatula or glass rod
- Beaker, 100 cm3
- Measuring cylinder, 250 cm3
- Measuring cylinder, 50 cm3
- Thermometer, 0–100 °C
- Stop clock
- Graph paper
- Access to a balance (1 d.p.)
- Access to water baths set at 20 °C, 30 °C and 37 °C (see note 2 below)
Chemicals
- Plain flour, 25 g
- Yeast suspension, 30 cm3 (see note 3 below)
- Sugar, 1 g
Health, safety and technical notes
- Read our standard health and safety guidance.
- If thermostatically controlled water baths are not available then large beakers of water maintained at the three temperatures can be used. The water temperature in each beaker needs to be monitored using a thermometer, and access to a supply of hot water, eg from a kettle, is needed to top up the beaker as the temperature falls.
- The yeast suspension is made by stirring 7 g of dried yeast with 450 cm3 of warm water. If fresh yeast is used it should not have been kept too long. Fresh yeast can be kept in the freezer for up to two months.
Procedure
- Add 25 g of flour to a beaker and then add 1 g of sugar.
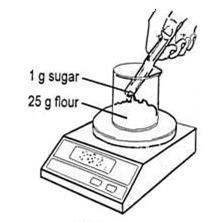
- Take 30 cm3 of the yeast suspension in a 50 cm3 measuring cylinder. Add the yeast suspension to the flour and sugar. Stir with a spatula or glass rod until a smooth paste, which can be poured, has been obtained.
- Pour the paste into a 250 cm3 measuring cylinder. Take great care not to let the paste touch the sides – this is very important.
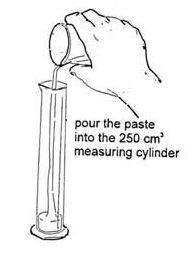
- Note the volume of paste in the cylinder. Place the cylinder in one of the water baths. Record the temperature and note the volume of paste every two minutes for about 30 minutes. A results table is useful here.
- Plot a graph to show how the volume of the dough increases with time. Plotting the results from groups, with the water baths at different temperatures, on the same graph will allow comparison of results.
Teaching notes
It is important that the paste does not touch the sides of the measuring cylinder when the students pour it from the beaker – this is easier said than done. One way of achieving this is to use a large plastic funnel which has had the stem cut off to leave a hole large enough for the paste to flow through the funnel. Alternatively, a suitable plastic drinks bottle could be cut to produce a wide mouthed ‘funnel’.
After 35–45 minutes the protein (gluten) breaks and carbon dioxide gas escapes.
Possible extensions for this activity could be to investigate the effect of substrate concentration (sugar), enzyme concentration and/or pH value on enzyme reactions.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet