Think critically, and you may come to some interesting conclusions
Examine the reaction of calcium metal with water from both a theoretical and a practical viewpoint.
Equipment
Materials per group
Fresh calcium metal, 0.5–1.0 g
Copper gauze to make a cage in which to sink the calcium, 60 mm × 60 mm
Equipment per group
Beaker (plastic if possible), 2 dm3
Thermometer, 0°–100° × 0.1°C
Safety screen or fume cupboard
Safety glasses
Health, safety and technical notes
- Read our standard health and safety guidance here.
- Wear eye protection.
- When attempting the practical route, students must have their proposals approved before starting on any experimental work.
- In contact with water calcium releases flammable gases, the use of a safety screen or fume cupboard should be mandatory.
Commentary
Various versions of the cycle are possible, but if the students invoke the electron affinity of the hydroxide radical, which is given in the brief, they should eventually arrive at the following:
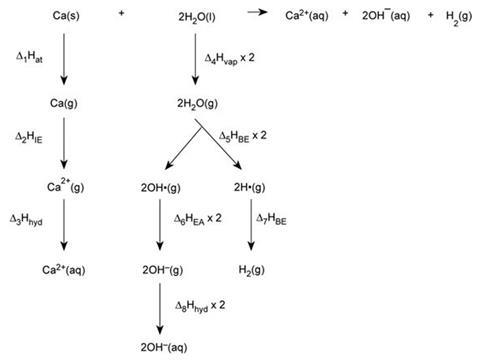
Energy values ΔH/kJ mol–1 (from data books and textbooks)
Δ1Hat = atomisation of Ca(s) = + 193
Δ2HIE = 1st and 2nd ionisation energies of Ca = +590 +1150 = + 1740
Δ3Hhyd = hydration energy of Ca2+ = –1650
Δ4Hvap = vaporisation of water × 2 = +44 × 2= + 88
Δ5HBE = bond energy O–H × 2 = +463 × 2 = + 926
Δ6HEA = electron affinity of OH•(g) = –176.5 × 2= – 353
Δ7HBE = bond energy H–H = –436 – 436
Δ8Hhyd = hydration energy of OH–(aq) = –460 × 2 – 920
– 412 kJ mol–1
Procedure
The experimental work is straight forward and gives results within 95% of the theoretical value using the following method.
Weigh about 100.00 g of deionised water in a 2 dm3 beaker. Carefully wipe the inside of the beaker to remove droplets above the water. Stand the beaker in a fume cupboard, insert a thermometer capable of reading to 0.1 °C in the water and leave to obtain a constant value. Weigh out about 0.5 g of calcium metal, choosing large pieces rather than smaller ones. Quickly empty the metal into the water and stir with the thermometer. If the calcium is fresh, the reaction will be complete in ca 90 s and the temperature increase is roughly 1.2 °C.
Some of the calcium floats, despite its density, because of the rapid evolution of hydrogen, hence some of the heat is lost to the air rather than to the water. To circumvent this problem during trialling, some students designed a copper cage to ensure that the calcium remained submerged. If this is done, a correction factor for the copper will be needed.
The accuracy of the experiment can be improved by using lagging and plotting cooling curves to find the theoretical maximum temperature rise.
Calculation
The experimental calculation is based on the energy change being related to the product of the mass of water x specific heat capacity of water x temperature rise.
Notes
This resource is part of a collection of problem-solving activities, designed to engage learners in small group work. Find out how to use these resources, and obtain a list of suggested ‘junk items’ here.
Downloads
Theory v practice - do they compare? - student handout
Experiment | PDF, Size 0.25 mbTheory v practice: do they compare? - teacher
Experiment | PDF, Size 0.3 mb
Additional information
The resources were originally published in the book In Search of More Solutions.
This activity is based on a problem written by Joe Burns.


















No comments yet