Let us take a closer look at the suitability of using molecular models to teach the determination of chemical formulae.
Using models to explain abstract concepts in chemistry is a very important teaching and learning tool, when students are trying to envisage what is happening at the sub-microscopic level. However, it is equally important that students understand what the models are actually trying to represent and appreciate the limitations of the models. If students fail to understand the limitations of the models it can reinforce or lead to further misconceptions.
Many of the simple models, such as coloured beads or plasticine can only be used to count the number of atoms and therefore only represent the chemical formula without giving away any other information.
Chemical Jigsaws can be used to represent ionic compounds.
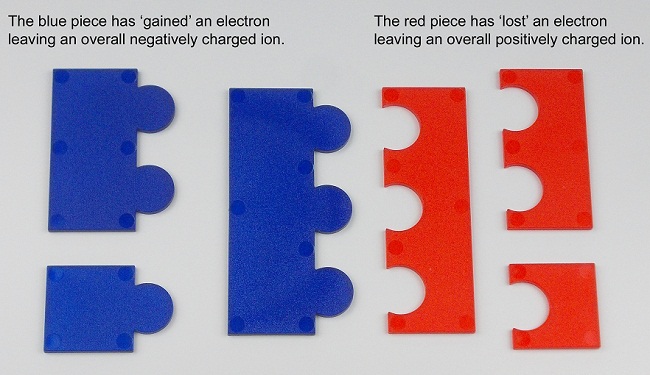
When using this model students often confuse the ‘anion’ and ‘cation’ but the model does help students to understand that the overall charge on a compound is zero.
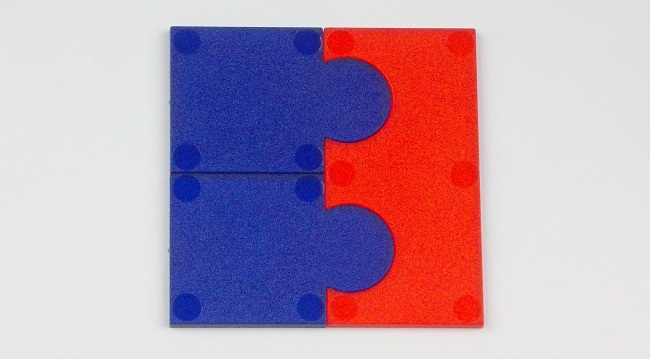
The above Chemical Jigsaw could be used to represent calcium chloride, CaCl2 (Ca2+, 2x Cl-). However, this model can also help to reinforce another common misconception. Students often think that the formula of an ionic compound represents a molecule (of calcium chloride etc) rather than that the formula indicates the ratio of positive and negative ions in a lattice structure.

So which is best; Chemical Jigsaws or Molymods?
It depends on the context of the lesson and your intended learning outcomes. They both have strengths and limitations, as shown in the table below.
| Strengths | Limitations | |
|---|---|---|
|
Chemical Jigsaws |
Can use to model both ionic and covalent substances. Helps students to write ionic formulae and appreciate that the overall charge is zero. Can link pieces to different groups on the periodic table eg Group 1 or 7. Helps students recognise displayed formulae as seen in textbook. |
2D, so cannot appreciate all bond angles. Does not show giant structures or crystal lattice. |
|
Molymods |
Models covalent substances. Giant structures such as diamond and graphite. 3D models. Show shape and bond angles. Good for modelling isomers as they can rotate around the single bond (and no rotation of double bonds). |
Cannot be used with ionic compounds. |
Another common difficulty that students often encounter, is understanding that the relative molecular (formula) mass is simply the sum of the relative atomic masses of the component elements. This can cause some students problems because they cannot physically weigh and compare the individual particles. Using formula cards can help to overcome this barrier to learning.
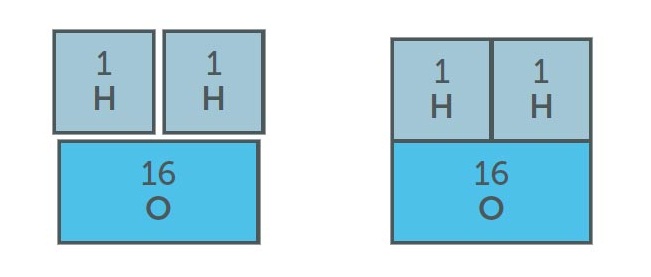
The relative molecular mass (Mr) = sum of Ar values of the component elements
Mr (H2O) = 1 + 1 + 16
= 18
Student activity
Follow the downloads for the ’Formula card student activity’ and ’Formula card template’ below.
Both elements of the student activity are adapted from Beyond Appearances: Students’ misconceptions about basic chemical ideas [2nd Edition (2004); Vanessa Kind, School of Education, Durham University].
Downloads
Formula card student activity
PDF, Size 0.12 mbFormula card template
Handout | PDF, Size 0.16 mb
Additional information
This activity appears in our Quantitative chemistry online CPD course, which includes over 113 different exercises and discussions like this to help you develop your students understanding of quantitative analysis. There are nine topics on the Conservation of mass, understanding Chemical equations and Using the mole which will take approximately nine hours to complete.
You can find further details about the structure of the course in the additional information below:
In this course you will learn about the building blocks that are required for a deep understanding of Quantitative chemistry.
After working through the course you will be able to:
- understand the core ideas in quantitative chemistry;
- explain how the core ideas of quantitative chemistry develop and progress throughout secondary education;
- identify common misconceptions and know how these can be addressed;
- confidently and competently teach aspects of quantitative chemistry to secondary aged students; and
- access a range of activities and resources to support students in their learning of quantitative chemistry.
Visit our teacher CPD pages to view our other courses.
Thank you Dorothy Warren and Maria Pack for authoring this course.
Description image used under license © Arno Jenkins/ Shutterstock.com.


















No comments yet