Use this practical to investigate the oxidation reactions of various alcohols with acidified potassium dichromate
In this experiment using a microscale well-plate, students add acidified dichromate(VI) to primary, secondary and tertiary alcohols to observe the difference in their oxidation reactions.
The experiment can be done by students in 20 minutes. The colour change of the dichromate(VI) indicates where reaction is occurring. Primary, secondary and tertiary alcohols can be distinguished by the rate of reaction, though no attempt is made to identify the products.
Students acidify the dichromate for themselves to emphasise the need for acid.
The alcohols can be stored in plastic pipettes and easily dispensed. The main difficulty for students arises from the fact that if the pipettes are squeezed too hard the alcohols come out of the pipette in a stream (because of their low surface tension). Students must handle the pipettes very carefully and some practice is required before proceeding with this experiment.
Equipment
Apparatus
- Eye protection
- Measuring cylinder, 5 or 10 cm3
- Well-plate (24 wells) – eg Sigma ref: M9655
- Glass rod
- Plastic dropping pipettes, x6
Chemicals
- Potassium dichromate(VI) solution, 0.1 M (TOXIC), 2 cm3
- Sulfuric acid, 1 M (IRRITANT), 1 cm3
- The following alcohols in dropping pipettes:
- Ethanol (HIGHLY FLAMMABLE) or Industrial denatured alcohol, IDA (HIGHLY FLAMMABLE, HARMFUL)
- Propan-1-ol (HIGHLY FLAMMABLE, IRRITANT)
- Propan-2-ol (HIGHLY FLAMMABLE, IRRITANT)
- 2-methylpropan-2-ol (HIGHLY FLAMMABLE, HARMFUL), or other tertiary alcohol
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Potassium dichromate(VI) solution, K2 Cr2 O7 (aq), (TOXIC) – see CLEAPSS Hazcard HC078c and CLEAPSS Recipe Book RB070.
- Dilute sulfuric acid, H2 SO4 (aq), (IRRITANT) – see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book RB098.
- Ethanol, C2 H5 OH(l), (HIGHLY FLAMMABLE or HIGHLY FLAMMABLE, HARMFUL if using IDA, industrial denatured alcohol) – see CLEAPSS Hazcard HC040A.
- Propan-1-ol, CH3 CH2 CH2 OH(l), (HIGHLY FLAMMABLE, IRRITANT) – see CLEAPSS Hazcard HC084A.
- Propan-2-ol, CH3 CH(OH)CH3 (l), (HIGHLY FLAMMABLE, IRRITANT) – see CLEAPSS Hazcard HC084A.
- 2-methylpropan-2-ol, (CH3)3 COH(l), (HIGHLY FLAMMABLE, HARMFUL) – see CLEAPSS Hazcard HC084B.
Procedure
- Take approximately 2 cm3 of potassium dichromate(VI) solution in a measuring cylinder and add about 1 cm3 of dilute sulfuric acid. Stir with a glass rod.
- Put 10 drops of the acidified potassium dichromate(VI) solution into each of the wells A1 – A4 and B2 (see diagram below).
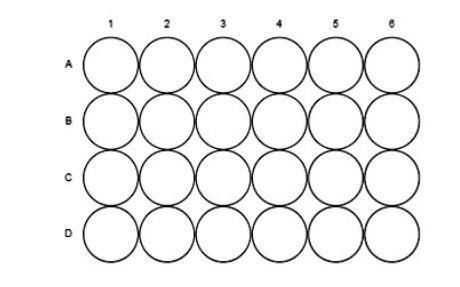
- Practice (over a sink) producing single drops of ethanol from the pipette.
- Add two drops of the alcohols to the wells as follows:
| Well number: | Alcohol |
|---|---|
| A1 | Ethanol |
| A2 | Propan-1-ol |
| A3 | Propan-2-ol |
| A4 | 2-methylpropan-2-ol |
- Observe the wells over the next 15 minutes and record any changes you see. Do not put any alcohol into well B2 – this well is used as a control.
Teaching notes
For the primary alcohols (ethanol and propan-1-ol), the dichromate turns green after a few minutes. The secondary alcohol (propan-2-ol) is slower. The tertiary alcohol (2-methylpropan-2-ol) is not oxidised at all. (Methanol could have been used in another well but, since this is slower than the other two primary alcohols, the differences are then not so obvious.)
Discussion could continue to the products of oxidation (aldehydes, then carboxylic acids for primary alcohols; ketones for secondary alcohols), and students could draw out the relevant alcohol and oxidation product structures.
Macro-scale experiments involving distillation and reflux of primary alcohols, to get aldehydes and acids respectively, may be useful to demonstrate the techniques involved, but seldom yield very satisfactory results in terms of identifying the products.
Further information
Chemguide provides a good summary of the theory behind the oxidation of alcohols.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
Health and safety checked, 2016

















1 Reader's comment