How well does the simple particle model of matter explain observations of changes of state? Watch these videos to assess how effective the model is and how you might apply it in your teaching.
The simple particle model can be used to explain physical changes such as changes of state (The particle model of matter is explained further in our full Developing and using models course).
Are there any exceptions to the rules or to the way in which the model is sometimes applied?
Using the particle model, explain what happens to the water particles when ice is heated so that it melts to liquid water and is then heated further until the water boils.
Watch the video clip and note down your observations.
Does the particle model explain your observations?
Yes, the particle model can explain the phase changes from solid → liquid → gas. In this example of the ice, the application of the model is straightforward. When ice is heated, the energy transferred to the ice increases the vibration of the water particles. This increased vibration breaks down some of the attractive forces between the water particles in the solid. The solid melts to give liquid water where the water particles can slide past each other easily.
Heating liquid water increases the motion of the particles still further. This results in more of the attractive forces between the water particles in the liquid breaking down. Given sufficient energy, the water particles are able to break completely free of the liquid. Water is now in the gaseous state where the water particles are in constant random motion.
Now use the particle theory to explain what happens when you heat solid sulfur until just before the liquid sulfur boils.
Watch the video clip and note down your observations.
Does the particle model explain your observations?
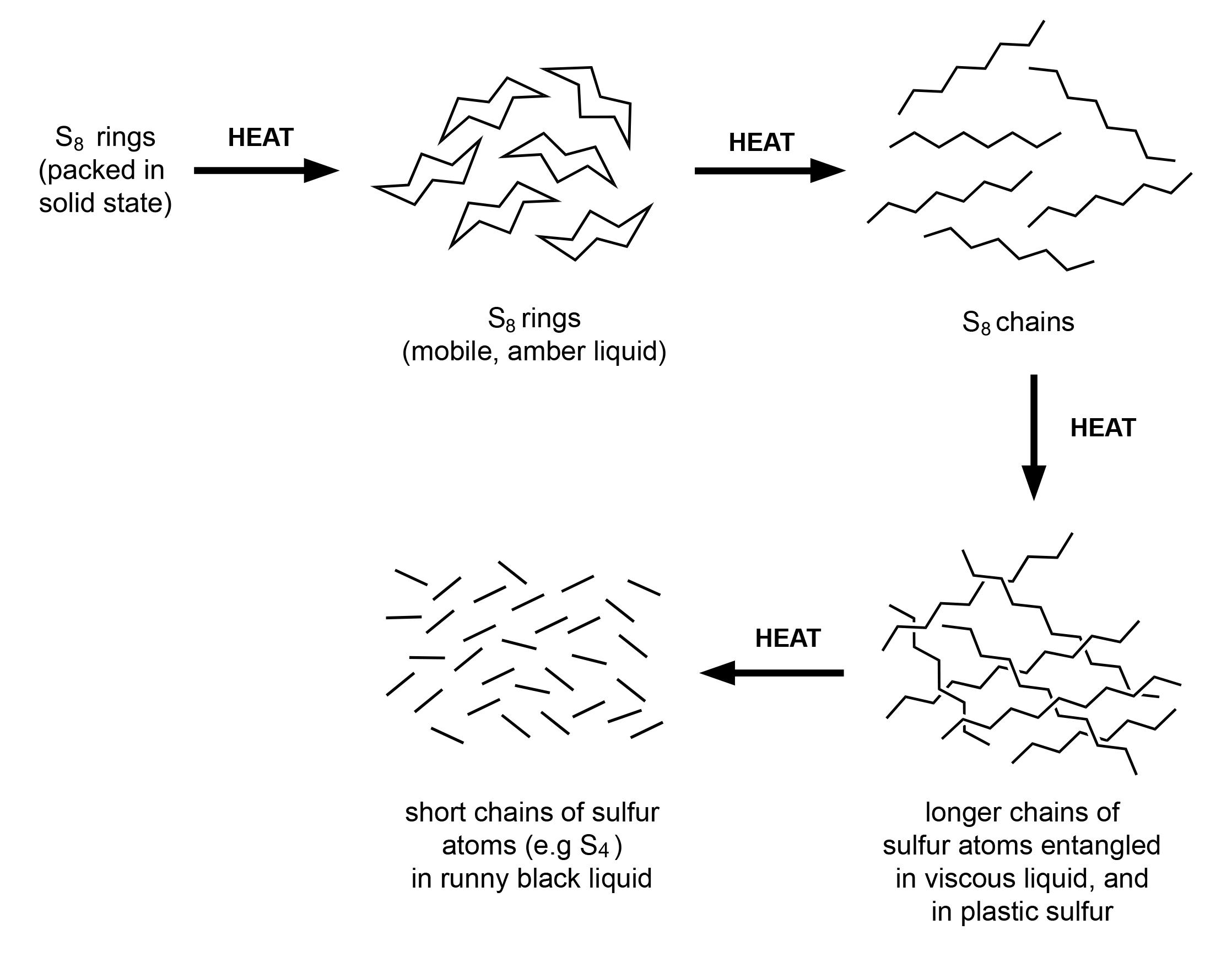
The particle model can explain the initial phase change from solid sulfur to liquid sulfur, as explained in the previous example with ice. However, with sulfur, there are some additional observations in the liquid state that need to be explained. The liquid changing from a runny liquid to a more viscous liquid and then becoming less viscous, as heating continued, was not expected.
In order to try and explain these observations in liquid sulfur, we need to modify the application of the particle model. These observations can be explained by first considering the breaking up of the sulfur rings.
Additional information
Sign up to our Developing and using models online CPD course for 51 different activities, discussions, techniques and development support like this.
The course includes five topics on how to use Scientific models and theories to Using models to explain scientific observations.
It will take approximately five hours to complete.
You can find further details about the structure of the course in the additional information below:
Our Developing and using models course offers insight into the steps of developing, using and assessing a model. These processes will be illustrated by examples, from a range of different chemical contexts such as the particle nature of matter, balancing equations, equilibria, structure and bonding.
After working through the full course you will:
- develop an understanding of the difficulties faced by students when trying to link macroscopic observations to microscopic explanations;
- appreciate how the key ideas of modelling develop and progress throughout secondary education;
- become more confident and competent in using models as a key idea in the teaching of chemistry to secondary aged students; and
- be equipped with a range of activities and resources to support students in developing their ideas about models.
Visit our teacher CPD pages to view our other courses.
Thank you to Dorothy Warren and John Walker for authoring this course.



























No comments yet