Build a solid foundation by securing the fundamentals
This is the second lesson in an introductory course for post-16 chemistry learners covering key ideas in order of scale. Find more information about the course here or download the ready-to-use worksheets.
How to use this resource
Before each lesson, ask learners to complete the preparation worksheet to revise knowledge from their 14–16 courses and introduce the topic for the lesson.
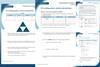
Then, get them to complete the student sheet during the lesson. It includes all key content and challenges misconceptions. Each student sheet has a scale and a Johnstone’s triangle diagram at the top. Use these to help learners think about the relative scale of different aspects of chemistry and connect their understanding of sub-microscopic, macroscopic and symbolic representations.
The book icon on the student sheets indicates that students will need access to learning materials e.g. textbook or online resources to support their learning.
Begin each lesson by checking learners have completed the preparation work. Share the answers and ask learners to mark their own worksheets as part of their independent work.
Topics in this lesson
Preparation worksheet – revision: atoms and subatomic particles; new content: counting subatomic particles.
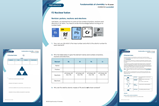
Student sheet – subatomic particles and the periodic table; isotopes; relative atomic mass.
Next lesson: F3 Nuclear fusion
Downloads
F2 Counting protons, neutrons and electrons preparation worksheet
Handout | PDF, Size 0.14 mbF2 Counting protons, neutrons and electrons student sheet
Handout | PDF, Size 0.38 mbF2 Counting protons, neutrons and electrons teacher notes
Handout | PDF, Size 0.54 mbF2 Counting protons, neutrons and electrons preparation worksheet
Editable handout | Word, Size 1.92 mbF2 Counting protons, neutrons and electrons student sheet
Editable handout | Word, Size 1.25 mbF2 Counting protons, neutrons and electrons teacher notes
Editable handout | Word, Size 0.76 mb


Fundamentals of chemistry | 16–18 years
- 1
- 2
- 3
 Currently reading
Currently readingF2 Counting protons, neutrons and electrons
- 4
- 5
- 6
- 7




















No comments yet