Reconsider how you teach this topic to ensure your 14–16 students overcome misconceptions
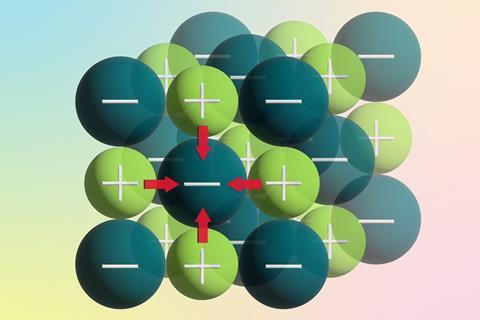
What do we want our students to say when we ask them to describe ionic bonding? Ideally, they would talk about the electrostatic attraction between oppositely charged ions within a giant lattice of alternating positive and negative ions. However, the way we’ve traditionally taught ionic bonding in pre-16 courses sometimes results in confusion. Students often discuss the electron transfer process that creates ions from the reaction between metal and non-metal atoms. This misconception can hinder their progress in more advanced courses. So, how can we help students better understand and distinguish these ideas?
Common misconceptions
Dot and cross diagrams are regularly used to explain ionic bonding. These show the transfer of the outer electron(s) from the metal atoms to fill the outer shell of the non-metal atoms. However, this transfer of electrons is what happens during the redox reaction between a metal and a non-metal, and is not a type of bonding.
The emphasis on dot and cross diagrams also leads to a reliance on the octet rule to explain why bonding occurs. This rule can lead to the idea that atoms are always trying to get a stable full outer shell of electrons. The full outer shell rule can be useful, but can also hinder students developing more sophisticated understanding if they see the rule as the driving force for reaction.
Resources for your classroom
See all of our supporting resources for ionic bonding at a glance in our structure and bonding resources package for learners aged 14–16.
- Read Beyond appearances by Vanessa Kind, from page 56 onwards, for a thorough analysis of the research that identifies misconceptions, as well as suggestions to tackle them.
- When teaching younger learners, highlight the importance of electrostatic forces of attraction with a classroom activity on modelling ionic bonds with plasticine.
- Use this ionic bonding probe to find out what your students think and facilitate discussion about their understanding.
- Uncover and challenge misconceptions about bonding with a toolkit of pre-prepared diagnostic and response activities on particles and structure.
Resources for your classroom
- Read Beyond appearances by Vanessa Kind, from page 56 onwards, for a thorough analysis of the research that identifies misconceptions, as well as suggestions to tackle them: rsc.li/3iCrrSp
- When teaching younger learners, highlight the importance of electrostatic forces of attraction with a classroom activity on modelling ionic bonds with plasticine: rsc.li/3UC3mIY
- Use a diagnostic probe on ionic bonding to find out what your students think and facilitate discussion about their misconceptions: rsc.li/3iz8jVo
- Uncover and challenge misconceptions about bonding with a toolkit of pre-prepared diagnostic and response activities on particles and structure: bit.ly/3FzxiRN
What you need to know
Students who go on to post-16 courses will study the energy transfers involved in forming ions. They will discover that the first ionisation enthalpy of a gaseous metal atom, such as sodium, is very endothermic. Atoms certainly do not ‘want’ to lose their outer electrons. An isolated metal atom is therefore far more stable with its outer electrons than as a positive ion. However, it is common for students to cling on to the idea that metal atoms lose their outer electrons because they are trying to get a full outer shell.
What about adding an electron to an isolated non-metal atom such as chlorine? This process, called the first electron affinity, is exothermic. The magnitude of this enthalpy change doesn’t make up for the highly endothermic removal of the electrons from the metal though. Also, transfer of a second electron to an outer shell is an endothermic process, due to the repulsion between the second electron and the already negative ion.
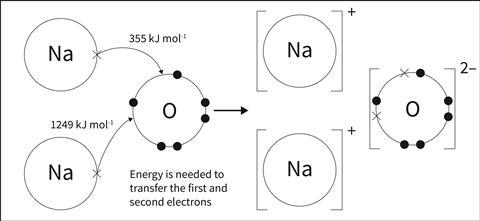
Whichever way you look at it, the electron transfer process shown by the dot and cross diagrams at pre-16 is not energetically feasible. Explaining that ionic bonding happens so that atoms get stable full outer shells just doesn’t fit the energetics. This begs the question, why do these ions form at all?
At post-16, students go on to look at Born–Haber enthalpy cycles, which describe the formation of ionic compounds from elements. Born–Haber cycles bring together all the energy-transfer processes involved. They show that to get to the isolated atoms depicted in the dot and cross diagrams, you need to break the metallic bonding in the solid metal and break the covalent bonding in the diatomic non-metal molecules. When this is added to the endothermic electron-transfer process, it means that a large amount of energy must be transferred to the reacting elements to form the gaseous ions.
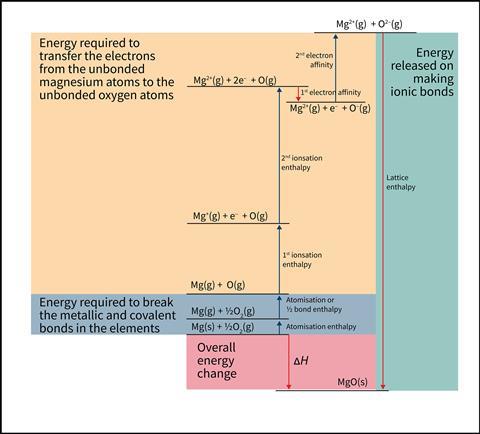
Suggestions for your teaching
With the current specifications and textbooks, it is not possible to totally separate the dot and cross diagrams from the description of ionic bonding itself. However, here are a few suggestions for a teaching order that might help students avoid some potential misconceptions.
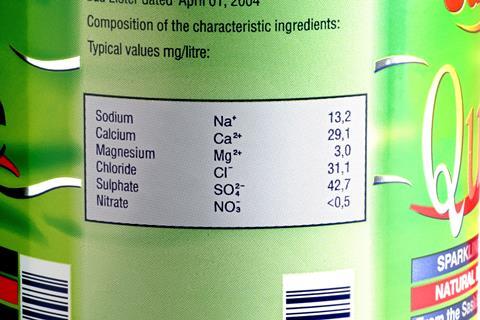
- Introduce ions and their formulas to students before they study bonding, ideally when they first meet atoms and molecules. Point out that some atoms and molecules may become charged and that these charged particles are called ions. As an interesting context, show them labels from bottles of mineral water.
- After teaching atomic structure, follow up by introducing dot and cross diagrams to show how atoms form ions and molecules. Keep the loss of electrons from metal atoms separate from the gain of electrons by non-metal atoms at this stage and don’t call it ionic bonding. Point out that atoms often end up with a full outer shell after forming molecules or ions. Highlight that we can use this rule to predict the number of covalent bonds or charges, but stress that this is not the full story.
- Focus on comparing the origin and strength of the electrostatic forces holding together different types of structure – giant metallic, giant ionic, giant covalent and simple molecular covalent. Don’t use dot and cross diagrams at this point. Cement the ideas of bonding and electrostatic forces in students’ minds.
- Use these ideas to position students to understand further topics that involve ions in redox reactions, such as reactions of group 1 and 7 elements, metal-acid reactions and electrolysis. That’s the time to show the transfer of electrons from metal to non-metal atoms, and the half-equations that go with them.














3 readers' comments