Boost your 16–18 students’ confidence with enthalphy changes and bonding
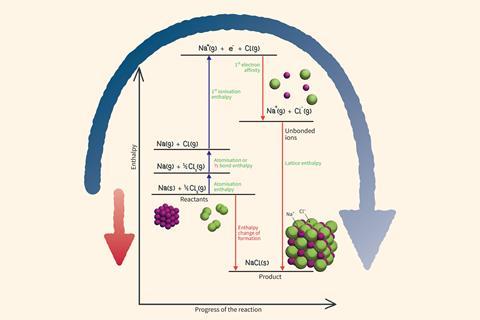
Born–Haber cycles are named after the two German scientists Max Born and Fritz Haber. The cycles were originally developed to calculate the lattice enthalpy of an ionic compound using Hess’s law. For this you need to know the standard enthalpy change of formation as well as the various enthalpy changes needed to make the gaseous ions from the elements in their standard states.
You can also use these cycles to compare which processes contribute most significantly to the overall stability of the ionic compound.
Download this
Infographic poster and fact sheet, student sheet and teacher notes, presentation and toolkit for student activity. Display the poster in your classroom or on a projector. Alternatively print it and use as a handout.
Use the accompanying activity to provide your 16–18 learners with plenty of practice.
How to draw a Born–Haber cycle for sodium chloride
It’s helpful to draw the cycle as an enthalpy level diagram so that the endothermic and exothermic processes are easily distinguished. Follow these five steps to draw the cycle for sodium chloride.
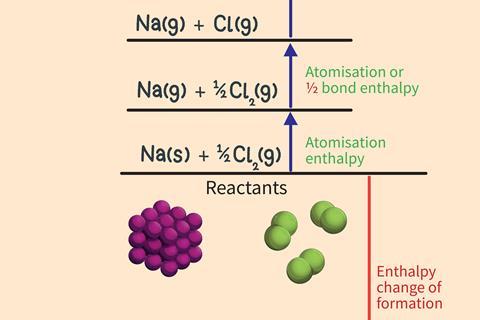
Step 1
Break the existing metallic and covalent bonding in the elements to form gaseous atoms of the metal and non-metal.
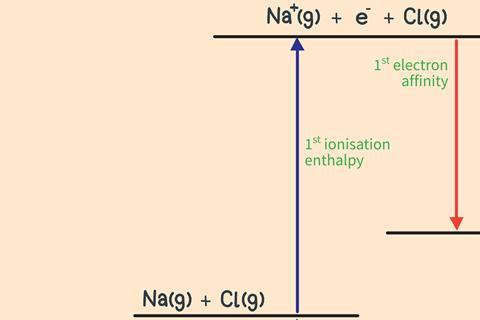
Step 2
Remove the outer electron from the gaseous metal atoms. This is endothermic as you have to work against the attraction of the nucleus to remove an electron.
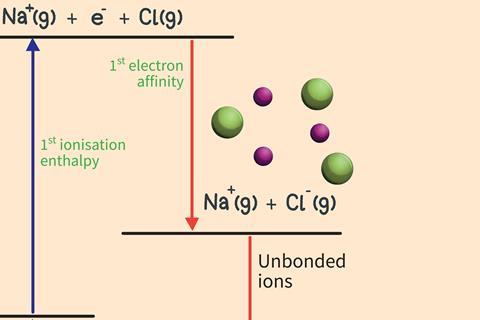
Step 3
Transfer the electron removed from the metal to the gaseous non-metal atoms. This is exothermic as the attraction of the nucleus pulls the additional electron towards the atom.
Step 4
Bring the gaseous ions together to form the solid ionic lattice. This is highly exothermic due to the strong attraction between the ions throughout the crystal.
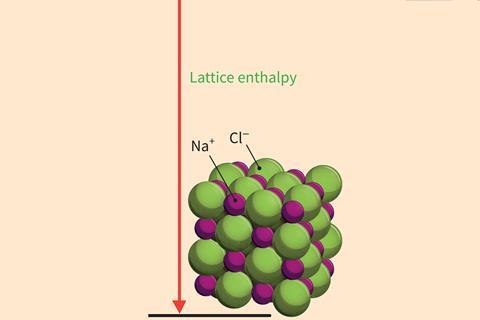
Step5
Complete the cycle by directly connecting the starting elements to the solid ionic compound.
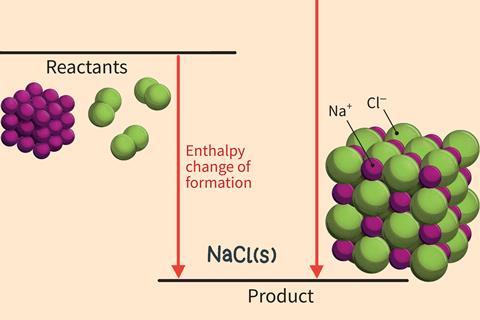
Larger cycles can also be drawn for the formation of compounds involving 2+ and 2- ions.
More resources
- Stimulate and challenge your students with these sudoku-style puzzles, including a puzzle for the Born–Haber cycle for magnesium chloride and the Born–Haber cycle for sodium chloride.
- Discover more ideas about how to teach energy and change post-16 with this CPD article.
- Test your learners’ understanding of thermodynamics with this Starters for 10 resource, which includes questions about Born–Haber cycles.
- Develop your skills even further with our free Energy and change CPD course, which includes a module on Born–Haber cycles.
Drawing cycles involving 2+ or 2- ion charges
All Born–Haber cycles have the same overall structure but you need to modify them if more than one electron is transferred between each metal and non-metal atom.
If the metal atom loses two electrons (eg Mg2+), then add the second ionisation energy to step 2 after the first ionisation energy.
If the non-metal atom gains two electrons (eg O2-), then add the second electron affinity to step 3. Note that, unlike the first electron affinity, it will be endothermic due to repulsion between the second electron and the negative ion.
If the formula of the ionic compound has a 1:2 ratio between elements (eg MgCl2 or Na2 O), then you must double the relevant enthalpy changes in steps 1, 2 or 3.
All illustrations © Dan Bright
Want more …
Step-by-step guides?
Try these articles with resources for 14–16 learners:
Poster of cycles?
Check out these articles with resources for 11–14 learners:
Downloads
EiC Born-Haber cycle poster
PDF, Size 0.89 mbEiC Born-Haber cycle fact sheet
Editable handout | Word, Size 0.45 mbEiC Born-Haber cycle fact sheet
Handout | PDF, Size 0.13 mbEiC Born–Haber cycle student worksheet
Editable handout | Word, Size 0.45 mbEiC Born–Haber cycle student worksheet
Handout | PDF, Size 0.21 mbEiC Born–Haber cycle teacher notes and answers
Editable handout | Word, Size 0.85 mbEiC Born–Haber cycle teacher notes and answers
Handout | PDF, Size 0.64 mbEiC Born–Haber cycle slides
Presentation | PDF, Size 1.19 mbEiC Born–Haber cycle animated slides
Presentation | PowerPoint, Size 1.14 mbEiC Born–Haber cycle interactive toolkit
Editable handout | Word, Size 0.57 mbEiC Born–Haber cycle interactive toolkit
Handout | PDF, Size 0.31 mb














3 readers' comments