Teaching the structure and bonding of carbon allotropes can be a little dry; you can spice things up a little by showing some of the key properties of graphite, dramatically destroying a pencil in the process.
Kit
- HB pencil, sharpened at both ends to approximately 5 cm
- Power supply unit delivering at least 5 A and 20 V. The power supply I use delivers 8 A and 25 V
- Suitable wiring
- Crocodile clips
- Heat resistant mat
- Ammeter (rated to 20 A)
- Voltmeter (rated to 20 V)
- Clamp stands
Preparation
You will need to sharpen a pencil at both ends. You might like to use an old pencil, but generally I find that by the time one has reached the right length (less than about 8 cm) through regular use, it has been through too many knocks and drops to be of use: the structural integrity of the graphite inside is paramount.
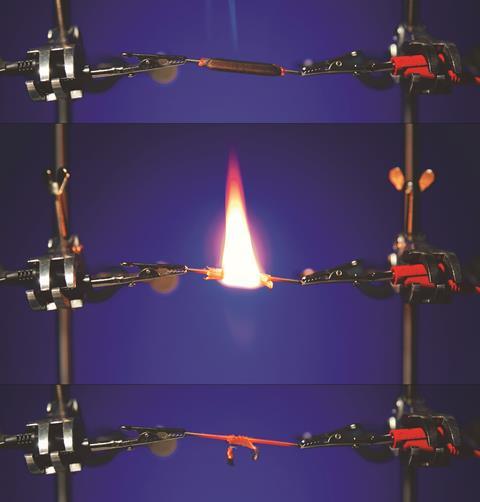
Working in a fume cupboard over a heat-resistant mat, suspend the pencil by its two ends from clamp stands using the crocodile clips, ensuring good electrical contact with the lead. Connect the ammeter in series with the power supply and the voltmeter across the pencil.
In front of the class
With a pencil of this type, if you set the power supply to deliver 1–1.5 A and 16–17 V, you should find after about 10 seconds thin trails of smoke begin to form from the two ends where the graphite meets the wood. Eventually the wood around the pencil reaches its ignition point and burns away to reveal the red hot graphite glowing beneath, thus showing both the conductivity and unusually high melting point of graphite.
Teaching goal
This activity coordinates nicely with Keith Taber’s resource The melting temperature of carbon, which helps students overcome difficulties associated with rationalising the structure of graphite, given its simple formula; many think of it as being monatomic like a noble gas.
Before showing the demo, I hand out the first sheet of the resource, in which students are asked to consider the pattern in melting points of several simple molecular elements. Using this pattern, they predict the melting point of carbon to be approximately 10–15 K (which it obviously isn’t). I gather them around and point out that pencil lead contains graphite and it obviously has not melted by room temperature. Making links to other molecular structures, we predict that it should still melt at a fairly low temperature and not conduct electricity. Students are often surprised and impressed by the spectacle as they see the wood burn away to reveal the red hot core, which is obviously carrying a current. The second sheet invites students to consider the type of structure in different elements and explain how graphite’s melting point can be so high.
This demonstration can be supported by a video model of C60 being heated. Several such clips are available on YouTube. Once I’ve introduced the students to the C60 structure, from a simple molecular viewpoint, we predict a lower melting point. (Technically, it will sublime at about 900 K, but I do my best to keep things only as complicated as needed for the discussion at hand).
As the video progresses, it becomes clear that the fullerene structure itself is maintained at very high temperatures and only begins to collapse above about 4500 K. This helps students to distinguish between simple molecular behaviour and macromolecular behaviour. I would love to find a similar simulation that models dynamics in 10 or more C60 molecules so the behaviour of the molecule could be more easily distinguished from the behaviour of the atoms. Perhaps one of our readers might be able to help?
Safety
- Wear eye protection.
- Working in a fume cupboard reduces the risk that students or staff will inhale potentially toxic fumes from the paint on the pencil burning. The use of pencils made from recycled plastic or similar materials would not be suitable for this demonstration. The large amount of smoke produced may reduce the life of the pre-filter in filtration fume cupboards. By using a plain wood pencil, or by scraping the paint off, the demo can be done in the open laboratory.
- The pencil and clamp get hot! If the pencil is held in a clamp, the jaws should not be lined with cork, rubber or any other material.
- Do not exceed 20 V.
- As a non-standard activity, this demonstration is not covered by model risk assessments; schools are likely to need a special risk assessment, which can be obtained from CLEAPSS by members.


















No comments yet