In this investigation, students use a spirit burner to burn various alcohols while measuring and comparing the amount of heat energy produced
This experiment is suitable for pre-16 students, possibly as an introduction to a topic on fuels. It can be taken further if used with post-16 students who can calculate values for enthalpy changes of combustion, with subsequent discussion about heat losses and incomplete combustion.
The alcohols should be provided in labelled spirit burners ready to use. If each group investigates one alcohol, the experiment can be done in around 20 minutes. It is better if each spirit burner is used by more than one group of students. Variation of results will add substance to a discussion about errors.
Equipment
Apparatus
- Eye protection
- Retort stand and clamp
- Conical flask, 150 cm3 or larger
- Measuring cylinder, 100 cm3
- Thermometer (–10 °C to +110 °C)
- Access to balances, preferably several, to avoid queuing
- Access to spirit burners with wicks and caps, containing the alcohols listed (note 1)
Apparatus notes
- Suitable spirit burners are hard to come by. Ideally they should be small, with a capacity of 50 cm3 or less. Pictures and information in suppliers’ catalogues can be misleading. It is important that the wick fits tightly in the wick holder and that the wick holder fits tightly in the burner. If capacity is more than 50 cm3, reduce it, for instance by packing with mineral wool, or partially filling with epoxy. Refer to CLEAPSS L195 ’Safer chemicals, safer reactions’. One possible source is: A.J.Cope & Son Ltd, Unit 10, Cliffside Trade Park, Motherwell Way, Grays, Essex, RM20 3XD.
Chemicals
- Methanol
- Ethanol
- Propan-1-ol
- Propan-2-ol
- Butan-1-ol
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Methanol, CH3OH(l), (HIGHLY FLAMMABLE, TOXIC) – see CLEAPSS Hazcard HC040b. Methanol is volatile and has a low flash point.
- Ethanol, CH3CH2OH(l), (HIGHLY FLAMMABLE) – see CLEAPSS Hazcard HC040A. Ethanol is volatile and has a low flash point.
- Propan-1-ol, CH3CH2CH2OH(l), (HIGHLY FLAMMABLE, IRRITANT, HARMFUL) – see CLEAPSS Hazcard HC084A. Propan-1-ol is volatile and has a low flash point.
- Propan-2-ol, CH3CHOHCH3(l), (HIGHLY FLAMMABLE, IRRITANT, HARMFUL) – see CLEAPSS Hazcard HC084A. Propan-2-ol is volatile and has a low flash point.
- Butan-1-ol, CH3CH2CH2CH2OH(l), (HIGHLY FLAMMABLE, IRRITANT, HARMFUL) – see CLEAPSS Hazcard HC084B. Butan-1-ol is volatile and has a low flash point.
Procedure
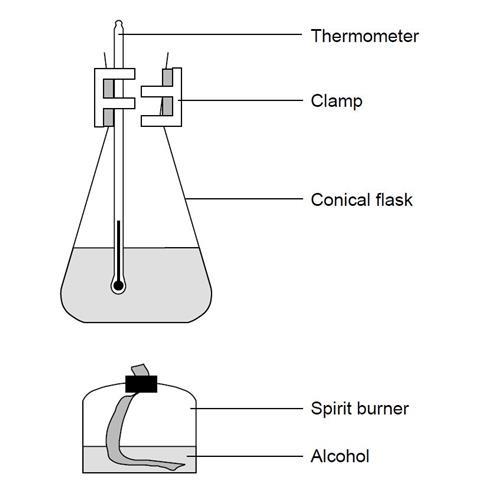
- Measure 100 cm3 of cold tap water into a conical flask.
- Clamp the flask at a suitable height so that a spirit burner can easily be placed below.
- Weigh the spirit burner (and cap) containing the alcohol and record this mass and the name of the alcohol.
- Record the initial temperature of the water in the flask.
- Place the spirit burner under the flask and light the wick.
- Allow the alcohol to heat the water so the temperature rises by about 40 °C.
- Replace the cap to extinguish the flame.
- Reweigh the spirit burner and cap, and record this mass.
- Work out the mass of alcohol used.
- Using a fresh 100 cm3 of cold tap water, repeat the experiment with another alcohol.
Teaching notes
Get the class to record and share the results. Do not be surprised if groups get different answers for a given alcohol. Heat losses will almost certainly vary considerably.
Subsequent discussion depends on the level of the students’ experience.
Student questions
Here are some possible questions to ask students:
- Which alcohol produces the most energy per gram?
- Which alcohol produces the most energy per mole?
- Write equations for the complete combustion of each alcohol.
- Propan-1-ol and propan-2-ol are isomers (same molecular formula, different structures). Do they produce the same amount of heat on combustion?
- Does all the heat produced by combustion go into raising the temperature of the water?
- Is it possible that combustion may be incomplete, giving carbon monoxide among the products?
- Alcohols can be used as a substitute for hydrocarbon fuels, and so methods of producing alcohols are very important. What process converts sugar into alcohol and carbon dioxide?
Notes on questions
- On question 6, stress the dangers accompanying the production of carbon monoxide.
More resources
Add context and inspire your learners with our short career videos showing how chemistry is making a difference.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















2 readers' comments