Various experiments to observe the effects of detergents and soaps on the surface tension of purified and hard water
A fine insoluble powder, such as talcum powder, is sprinkled on a clean water surface in a beaker, a drop of detergent or soap solution added to the centre, and the effect observed as the surface tension of the water is changed. This can be repeated with other powders after cleaning the beaker and using fresh water samples. A needle can be carefully floated on a clean water surface and the effect of adding detergent or soap observed. Finally the same experiments can be repeated using samples of hard water to compare the effects.
This is a series of quick, simple, class experiments which can be extended or shortened as desired. Each experiment should take no more than two minutes, though the cleaning of the beaker between experiments may take up more time than expected. If a full range of experiments is desired, the time taken could amount to 30 minutes, but this may not be justified in terms of the learning objectives concerned.
Equipment
Apparatus
- Beaker (250 cm3)
- Glass stirring rod
- Clean sewing needle (note 1)
Apparatus notes
- The sewing needle should be a fine needle, and for safety issued to students with the pointed end inserted into a piece of card bearing a safety warning about handling the needle.
Chemicals
- Talcum powder, in pepper pot or similar dispenser
- Other powders (see technical notes)
- Liquid detergent in a dropping bottle
- Liquid soap in a dropping bottle
- Access to a supply of purified water (distilled or deionised), about 1 dm3 per working group
- Access to a supply of hard water
Health, safety and technical notes
- Read our standard health and safety guidance
- Other powders – Any powders used other than talcum powder, such as lycopodium powder or carbon powder, should be subject to a risk assessment. Lycopodium powder is a potential allergen.
- Liquid detergent – Any washing-up liquid or multipurpose detergent will suffice.
- Liquid soap - Genuine liquid soap or soap flakes from which the liquid can be made, is increasingly difficult to obtain. Wanklyn’s and Clarke’s soap solutions should still be available from chemical suppliers. Lux soap flakes are ideal for making liquid soap if you can source them. Granny’s Original and other non-branded soap flakes work fine but need to be used in solution as soon as they are made. They do not form a stable emulsion and precipitate out overnight. Note that most liquid hand washes are based on the same detergents as washing-up liquids and do not contain soap. To obtain soap solution from soap flakes – dissolve soap flakes (or shavings from a bar of soap) in ethanol – use IDA (Industrial Denatured Alcohol) (HIGHTLY FLAMMABLE, HARMFUL) – see CLEAPSS Hazcard HC040a and CLEAPSS Recipe Book RB000. Do not dilute with water.
- Hard water – A supply of hard water can be made by stirring solid calcium sulfate into a large volume of tap water, allowing to stand for some time then, after the undissolved solid has settled out, decanting the clear solution into a container suitable for students to collect their samples as required. Label as ‘Hard Water’. Allow about 1 dm3 for each working group in the class.
Procedure
- Half fill the beaker with purified water.
- Sprinkle the water surface carefully with a fine layer of powder.
- Add one drop only of detergent in the middle of the water surface. Observe what happens. Does the talcum powder stay on the surface, or does it sink?
- Clean the beaker thoroughly, half-fill again with purified water, and repeat steps two and three using a drop of liquid soap instead of detergent. Compare what happens to what happened in the previous experiment.
- Repeat steps three and four, only this time use hard water instead of purified water. Are the results different from those obtained with purified water? If so, in what ways?
- Other powders may be available to test instead of talcum powder, to see whether the type of powder makes any difference. If you do test any of these, what differences do you find?
- Again using a clean beaker with purified water, try to float a fine sewing needle on the surface by carefully lowering it into the beaker, avoiding breaking the surface with your fingers, and dropping it from as close above the surface as possible. Once you have a needle floating, add a small drop of detergent to the water, but away from the needle. What happens?
Teaching notes
This series of brief experiments on the surface tension of water, and the effects of detergents and soaps on this, can serve as an introduction to the phenomenon of surface tension, with a discussion of results leading into simple theory. Alternatively, it could be used to illustrate prior teaching of the topic, leading to discussion of what is happening when detergents and soaps are added, including the differences found with hard water.
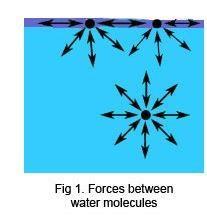
There is a net force of attraction between the molecules of water (or any other liquid) holding the molecules together. For a molecule in the middle of the liquid, these forces, acting equally in all directions, more or less balancing out. For a molecule in the surface layer of the liquid, the forces do not balance out, and all the molecules in the surface layer are pulled towards each other and towards the bulk of the liquid. This brings these molecules closer to their neighbours until increasing forces of repulsion create a new balance, and gives rise to the phenomenon of surface tension.
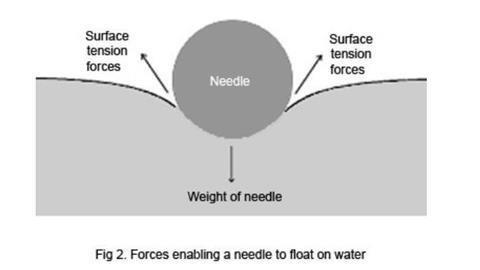
When an object falls onto the surface, it has to push the water molecules apart. If the effect of the weight of the object is insufficient to match the attractive forces between molecules in the surface layer, the object will not enter the surface. Careful observation of the floating needle will show that the water surface is bent down under the weight of the needle, the surface tension causing it to behave as if the needle was supported by a flexible skin.
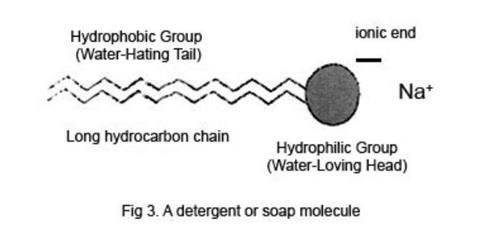
Molecules of most detergents and soaps are long chain hydrocarbon molecules with an ionic group at one end, usually carrying a negative charge, thus making it an anion. This charge is balanced by the opposite charge of a soluble cation, for example Na+. The long hydrocarbon chains do not interact well with water molecules, and many of them are effectively ‘squeezed out’ to the interfaces between the water and the air or the glass sides of the beaker. The effect of these molecules on the water surface is to considerably weaken the forces between water molecules there, thus lowering the surface tension.
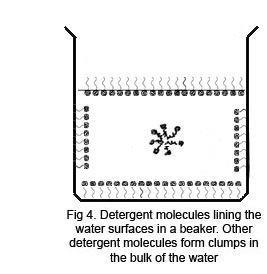
When the drop of detergent is added to the powdered surface, the initial effect is to draw the powder back to the edges very rapidly as the detergent molecules form their own surface layer with a lower surface tension than the water. As the detergent gradually mixes with the water, the powder begins to sink, and a needle will now pass through the surface with ease under its own weight. However, if lycopodium powder is used, which is less dense than water, it remains at the edges. Other powders may clump into nodules if they are not wetted by the detergent solution.
In hard water there is a significant concentration of calcium, Ca2+, and/or magnesium, Mg2+, cations. These cations form an insoluble compound with soap anions, so instead of forming a surface layer, they are precipitated out, leaving the surface tension largely unchanged.
2COO−(aq) + Ca2+(aq) → (COO)2Ca(s)
However, the calcium and magnesium salts of many detergent molecules are soluble, so detergents lower the surface tension of hard water.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet