Use this demonstration to illustrate how methane can create an explosive mixture with the oxygen in air.
In this simple experiment, students observe as a large tin fitted with a press-on lid and a glass chimney is filled with methane. When the gas is lit at the top of the chimney, the flame burns down and causes the methane–air mixture in the tin to change composition. After a while, an explosive mixture is reached and the lid of the tin is blown off with a loud bang.
This demonstration can be used for fun – eg at open days – or to provide an striking illustration of how a flammable gas can combine with air to create a mixture with explosive properties. The experiment can also be linked to domestic gas explosions, along with this related demonstration using hydrogen to create a controlled explosion in a plastic drink bottle.
A darkened room will heighten visibility of the explosion flame.
The time for carrying out the demonstration should be about five minutes.
Equipment
Apparatus
- Eye protection for the demonstrator
- Safety screens, x2
- A large tin with a press-on lid (see note 4 below)
- Glass tubing, 2–3 cm in diameter, 30–50 cm long
- Epoxy resin adhesive (eg Araldite)
- Length of rubber tubing
- Boss, clamp and stand
- Wooden splints
Chemicals
- Methane (natural gas) supply (EXTREMELY FLAMMABLE)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Methane (natural gas), CH4(g), (EXTREMELY FLAMMABLE) – see CLEAPSS Hazcard HC045a.
- A catering size (500–750 g) instant coffee tin works well. Make a small hole about 1 cm in diameter in the base of the can. Make a larger hole halfway up the side of the tin to take the glass tubing. Use epoxy adhesive to glue the glass tube in place – see the diagram below.
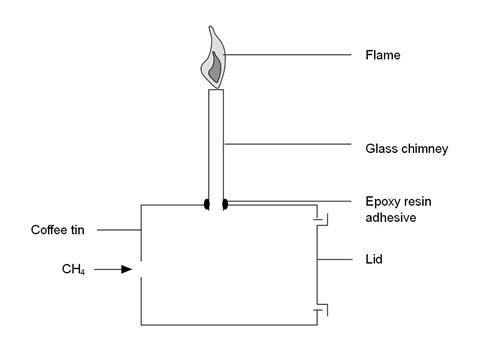
Procedure
- Put the lid on the tin, place it on its side and use the glass chimney to clamp it in position so that the lid is facing away from the class – see the diagram above. Place a safety screen between the tin and the class and add protection for the demonstrator with a second screen.
- Insert the rubber tube attached to the gas source into the hole in the base of the tin and fill the tin with methane. Allow at least a minute to ensure that the methane has displaced all the air in the tin.
- Turn off the gas supply and remove the tubing. Without delay use a lighted splint at arm’s length to ignite the gas emerging from the chimney. It will initially burn with a yellow luminous flame. This will change to a blue flame as more air is drawn into the tin.
- After a short while the flame will start to descend the chimney. As it reaches the bottom, the gas mixture in the can will explode, blowing the lid off the can. The explosion is fairly gentle and the lid must not be too tightly in place or it will not be blown off.
Teaching notes
The reaction is the combustion of methane to form carbon dioxide and water:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l), ΔH = –890 kJ mol–1
The similarity of the flame to the flames of a Bunsen burner with the air hole open and shut should be pointed out. The flame descends the chimney because the combustion reaction is using up gas faster than the gas is rising up the chimney.
The energy released appears as heat, light, sound and kinetic energy (of the flying lid), similar to the situation in an internal combustion engine. Methane–air mixtures have quite narrow explosive limits (4–17 mol %), whereas hydrogen-air mixtures are explosive over a much broader range (4–77 mol %).
The source of the energy produced by the reaction could be discussed in terms of the breaking and making of bonds.
This demonstration will not work with other domestic gases, such as propane and butane, because they are denser than air. This difference also affects how these gases behave in the event of leaks. Methane (natural gas) would concentrate near the ceiling or move upstairs and could escape through open high windows. Propane and butane would sink, building up at floor level, and could migrate down stairs or into cellars.
Many mining disasters, especially coal mining, have been caused by explosion of methane–air mixtures. Methane levels have to be constantly monitored. Canaries and the Davy safety lamp have been used in the past.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet