11–14 chemistry: ideas, resources, and misconceptions to look out for
11–14 chemistry: David Paterson suggests ideas, resources, and misconceptions to look out for
How did knowledge of chemical mixtures prevent a major incident in the London Underground?
When an accidental leak of wet, quick-drying concrete flooded London’s Victoria underground station control room, engineers’ quick thinking and chemical knowledge prevented total disaster. They mixed large amounts of sugar into the concrete, slowing down the setting process, and allowing them time to clear up the spill.
When an accidental leak of wet, quick-drying concrete flooded London’s Victoria underground station control room, engineers’ quick thinking and chemical knowledge prevented total disaster. They mixed large amounts of sugar into the concrete, slowing down the setting process, and allowing them time to clear up the spill. (Read the full story: rsc.li/EiC-concrete)
Mixtures and solutions are a common occurrence in our everyday lives. They are the air we breathe, the food and drink we consume and the fabrics we wear. By studying how chemists distinguish pure substances from mixtures and solutions, students will start to appreciate how matter is organised at the atomic level. With this knowledge, we can manipulate matter to improve our health and quality of life.
What do students need to know about mixtures and solutions?
- Mixtures are materials that contain two or more chemical substances dispersed among each other (mixed together).
- If no chemical reaction occurs when two materials are mixed, they form a mixture. The chemical properties of the components don’t change.
- Mixtures can be separated by physical methods.
- There are two general types of mixtures: homogeneous and heterogeneous.
- Homogeneous mixtures: the particles of the substances are mixed together (there is no clumping of the particles) – eg air.
- Solutions are homogenous mixtures: particles of one substance (the solute) are mixed together with the particles of another substance (the solvent) – eg salty water.
- Heterogeneous mixtures: large aggregations (clumps) of the substances are mixed together – eg emulsions like oil in water.
When students are learning how to use technical language correctly, the number of new words to learn and the precision of use required can daunt them. The word ‘stuff’ is a useful starting point: while it is not an accepted scientific term, it has tangible meaning for most students so is a useful linguistic stepping stone.
Students understand the world is made of stuff, and different stuff has different properties. Our job is to help them move beyond their view of the world based on their senses, to the submicroscopic world where different chemical substances are arranged in different ways. The Gatsby Science Enhancement Programme’s ‘Stuff and substance’ package provides lots of simple activities and animations.
Students understand the world is made of stuff, and different stuff has different properties. Our job is to help them move beyond their view of the world based on their senses, to the submicroscopic world where different chemical substances are arranged in different ways. The Gatsby Science Enhancement Programme’s ‘Stuff and substance’ package provides lots of simple activities and animations (bit.ly/2DoV2o1).
Ideas for the classroom

Using physical models provides students a hook for developing their understanding. For example, present them with a set of pots containing identical plain beads, identically sized but differently coloured beads, and beads of the same colour but different sizes.
After they have had a couple of minutes to look at them and discuss, pose the questions …
Which of the pots contain mixtures? How can you tell? What properties of the contents are you using to make this decision?
The students should identify the last two as mixtures, as they contain beads differing in size or colour. Ask students to present their reasoning to the class, and ask others to agree or disagree and explain why. Challenge those presenting sophisticated arguments to extend their reasoning. For example, for the third pot, ‘Is it enough to say the beads are different because they have different sizes? Would you make the same argument for apples of different sizes?’ This type of questioning can help students look beyond surface properties and consider whether there are other more important properties of objects and substances.
Repeat and extend the activity to more ‘chemical’ examples by presenting the students with sealed test tubes of salt, sand, salt and sand, and zinc pieces and copper turnings.
Want to try this activity with your class? Download the PowerPoint slides for a step-by-step, in-class guide (ppt or pdf).
Want to try this activity with your class? Download the PowerPoint slides for a step-by-step, in-class guide (rsc.li/2Gnctsm).
Once students are able to identify and explain mixtures, move on to the properties of mixtures. Students should understand that substances in mixtures retain their properties, and the properties of the mixture are a combination of these properties. Real-world examples can help students with these concepts. For example, sugar is sweet, water is wet, and a sugar solution is sweet and wet. The ‘Separating Mixtures’ activity in Learn Chemistry provides a useful hook for students’ understanding.
Once students are able to identify and explain mixtures, move on to the properties of mixtures. Students should understand that substances in mixtures retain their properties, and the properties of the mixture are a combination of these properties. Real-world examples can help students with these concepts. For example, sugar is sweet, water is wet, and a sugar solution is sweet and wet. The ‘Separating Mixtures’ activity in Learn Chemistry provides a useful hook for students’ understanding (rsc.li/2tIg0yJ).
Demonstrate making solutions by dissolving sugar in water, and the reverse by gently heating small volumes of the solution in evaporating dishes. Show miscible liquids (liquids that mix) and immiscible liquids (liquids that don’t mix) by mixing alcohol and water, and oil and water, respectively. Demonstrate other types of mixtures, such as suspensions, gels and foams, with flour in water, jelly and whipped cream.
Students will encounter many examples of mixtures throughout their chemistry studies, providing them regular opportunities to reinforce their understanding of mixtures and solutions.
For example:
- air is a homogeneous mixture that contains oxygen, nitrogen, argon and other gases;
- iron filings with sulfur powder is a commonly used heterogeneous mixture;
- salty water is a solution that contains particles of salt mixed with particles of water.
Encourage students to find their own examples from their own homes.
Need some inspiration for planning a lesson? Download this example lesson plan that incorporates these ideas (MS Word or pdf).
Need some inspiration for planning a lesson? Download this example lesson plan that incorporates these ideas (rsc.li/2Gnctsm).
Common misconceptions
Most ‘pure’ materials we come across in everyday life are actually mixtures of several chemicals. The difference between everyday purity (eg ‘100% pure orange juice’) and chemical purity (ie materials containing particles of only one chemical substance) causes considerable confusion. It is helpful to emphasise that learning science is as much about learning a new language as it is about learning concepts and skills.
When solutions form, many students think the mass of the solute is lost as it disappears into solution, although they will readily describe sugary water as tasting sweet. They may rarely recognise that the properties of a mixture are dependent on its exact composition.
Finally, students rarely recognise water (a very common solvent) as being particulate. Student-drawn representations of sugar dissolving in water often shows sugar particles in a continuous background of water.
Chemical misconceptions contains many useful ideas and worksheets to help diagnose students thinking. The ‘Elements, compounds and mixtures’ and ‘Mass and dissolving’ sections are particularly useful.
Chemical misconceptions contains many useful ideas and worksheets to help diagnose students thinking. The ‘Elements, compounds and mixtures’ (rsc.li/1pXL7iV) and ‘Mass and dissolving’ (rsc.li/2HuAT2F) sections are particularly useful.
Sample diagnostic question
You have a beaker of water that weighs 200 g. You add 10 g copper sulfate to the beaker of water. Initially, the copper sulfate solid can be seen in the water, but 10 minutes later the solid cannot be seen and the water turns blue.
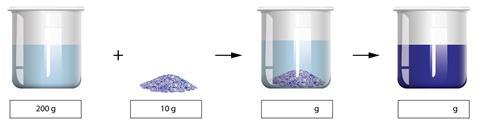
What is the mass of the beaker and its contents when the copper sulfate is first added? What is the mass when it has all finally dissolved in the water? Explain your answers.
Formative assessment
Students will not gain a proficient understanding of mixtures and solutions the first time they encounter these ideas. Regular formative assessment will help them to develop their understanding. For example, ask students to:
- write an explanation for describing a material as a mixture or a solution;
- draw a particle diagram of a substance, clearly distinguishing the different components;
- complete a short quiz on keyword definitions.
Then provide them with feedback and have them recomplete the task.
Progression to 14–16
The nature of chemical substances is a fundamental concept in chemistry, underpinning most of 14–16 chemistry. Concepts of elements, compounds, atoms and molecules will add to the understanding of mixtures and solutions, alongside separation techniques. These concepts will be reinforced and contextualised at 14–16, when students study materials such as crude oil and rock ores. Students will also need to understand the nature of chemical models. Consider refreshing your own knowledge of this area with the free ‘Developing and Using Models’ online course.
The nature of chemical substances is a fundamental concept in chemistry, underpinning most of 14–16 chemistry. Concepts of elements, compounds, atoms and molecules will add to the understanding of mixtures and solutions, alongside separation techniques. These concepts will be reinforced and contextualised at 14–16, when students study materials such as crude oil and rock ores. Students will also need to understand the nature of chemical models. Consider refreshing your own knowledge of this area with the free ‘Developing and Using Models’ online course (rsc.li/CPD-models).
Take-home points
- Mixtures are materials that contain two or more chemical substances mixed together (but not reacted together). Substances retain their chemical characteristics in mixtures.
- Substances described as ‘pure’ in everyday life are likely to be chemical mixtures.
- Listen to how students use the language and ideas of mixtures in the classroom, and help them rethink and rephrase as required.
- Students’ understanding of mixtures will be developed into their understanding of compounds, where mixed substances react together to create new substances.
Downloads
Mixtures - what do they look like?
PDF, Size 0.33 mbMixtures - what do they look like?
PowerPoint, Size 3.07 mbExample lesson plan
Word, Size 54.94 kbExample lesson plan
PDF, Size 50.98 kb















1 Reader's comment