Show your students how to find elementary information
The periodic table contains all the known elements. Elements are substances that contain only one type of atom. An atom is the basic building block for all matter – they’re not the smallest or simplest particles that exist, but they are the smallest, simplest parts of an element and they uniquely define each one, depending on their composition.
Different elements join together chemically to make compounds, but the periodic table only contains the elements. So, while water isn’t found in the table, the elements it is composed of, hydrogen and oxygen, both are.
Everything in the universe is made of different combinations of elements and the elements that make up our world originated in the stars. So, we really can say that we are made of stars!
Download this
Infographic poster and fact sheet, student worksheet, card sort and classroom presentation slides for organising elements activity. Display the poster in your classroom or on a projector. Alternatively print it and use as a handout.
Use the accompanying activity to organise unknown elements using their similarities, physical properties, appearance and reactivity. Walk in the footsteps of the chemists who organised the modern periodic table of elements. Learners can use the card sort included in the teacher notes or get the information from the presentation slides to build their own miniature table with just nine elements and three groups.
Reading and writing chemical symbols
Chemists spend a lot of time talking and writing about elements: what they’re like, how they react, how they change. Giving them symbols makes this process clearer, especially as these symbols are recognised all over the world, even if the names themselves vary in different languages.
When you write the symbol for an element, it’s really important that the first letter is a capital and the second letter (if it has one) is lower case. This means that, however many elements might be listed in an equation, you’ll always be able to distinguish between them.

Periodic tables generally display two numbers with each element. The smaller number is the atomic number. This is the number of protons, which is unique to each element and doesn’t change. The larger number is the relative atomic mass of an element – the higher the number, the greater its mass.
More elements could be added to the periodic table in the future. Four new elements were recognised in 2016, but they were made in a laboratory and can only exist for a short time.
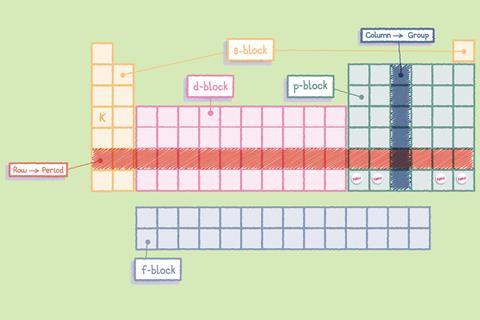
Using the table’s structure
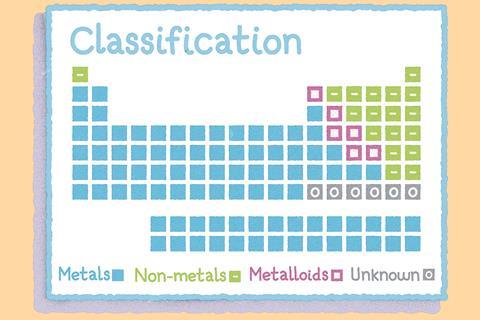
Within the table, the arrangement of the elements means that their properties vary periodically. As you move along each row or period, there are repeating patterns in the chemical and physical properties of the elements in each one. For example, you’ll find metals on the left-hand side and non-metals on the right.
Chemists can make predictions about elements based on where they are in the periodic table. Elements in the same column or group have similar properties and within each group their properties vary in a predictable way. For example, elements towards the bottom of the first group react more readily than the elements higher up in the group.
The block in the middle of the table, beginning in the fourth period, contains the transition elements. The transition elements are metals and often react with other elements to form brightly coloured compounds. They are usually relatively hard and are often used as catalysts (they help speed up chemical reactions, without being used up in the reactions themselves).
The periodic table is useful to chemists because of the way the elements are arranged but this didn’t happen quickly or by chance. Several scientists arranged the elements they knew about in different ways before Dmitri Mendeleev arranged them as they are today. This was impressive, because he left gaps for elements that hadn’t been discovered yet and he did this before we fully understood the structure and composition of atoms.
All illustrations © Dan Bright
Want more…
… posters and activities for your 11–14 learners?
Try these:
- Earth’s resources, with scaffolded calculations worksheet
- Earth’s structure, plus differentiated DART activity
- Rock cycle poster, plus Rock and roll game
- Water cycle poster, with structure strips
- Evaporation poster and practical activity
- Melting chocolate poster, with changes of state and mixtures worksheet
… articles and resources about the periodic table?
Try these:
- Use this poster, fact sheet and classroom activity looking at groups 1, 7 and 0 with your 14–16 learners
- Read why learners don’t need to memorise the periodic table.
- Find out how to help students use the periodic table as a learning aid.
- Read all about the most important village in chemistry and download a worksheet on isotopes.
- Discover a printable periodic table poster.
Downloads
Organising elements student worksheet
Handout | PDF, Size 0.16 mbOrganising elements teacher notes, printable cards and answers
Handout | PDF, Size 0.22 mbOrganising elements classroom presentation
Presentation | PDF, Size 0.37 mbOrganising elements student worksheet
Editable handout | Word, Size 0.44 mbOrganising elements teacher notes, printable cards and answers
Editable handout | Word, Size 0.44 mbOrganising elements classroom presentation
Presentation | PowerPoint, Size 2.54 mbEiC How to read the periodic table fact sheet
PDF, Size 97.17 kbEiC How to read the periodic table fact sheet
Word, Size 56.21 kbEiC How to read the periodic table poster
PDF, Size 0.45 mb









1 Reader's comment