Boost your 11–14 students’ knowledge and understanding of this separation technique with this poster, fact sheet and practical activity
Next time it rains, look at the puddles left behind. Shallow puddles disappear quickly while deeper ones stay around longer. You’re watching evaporation in action.
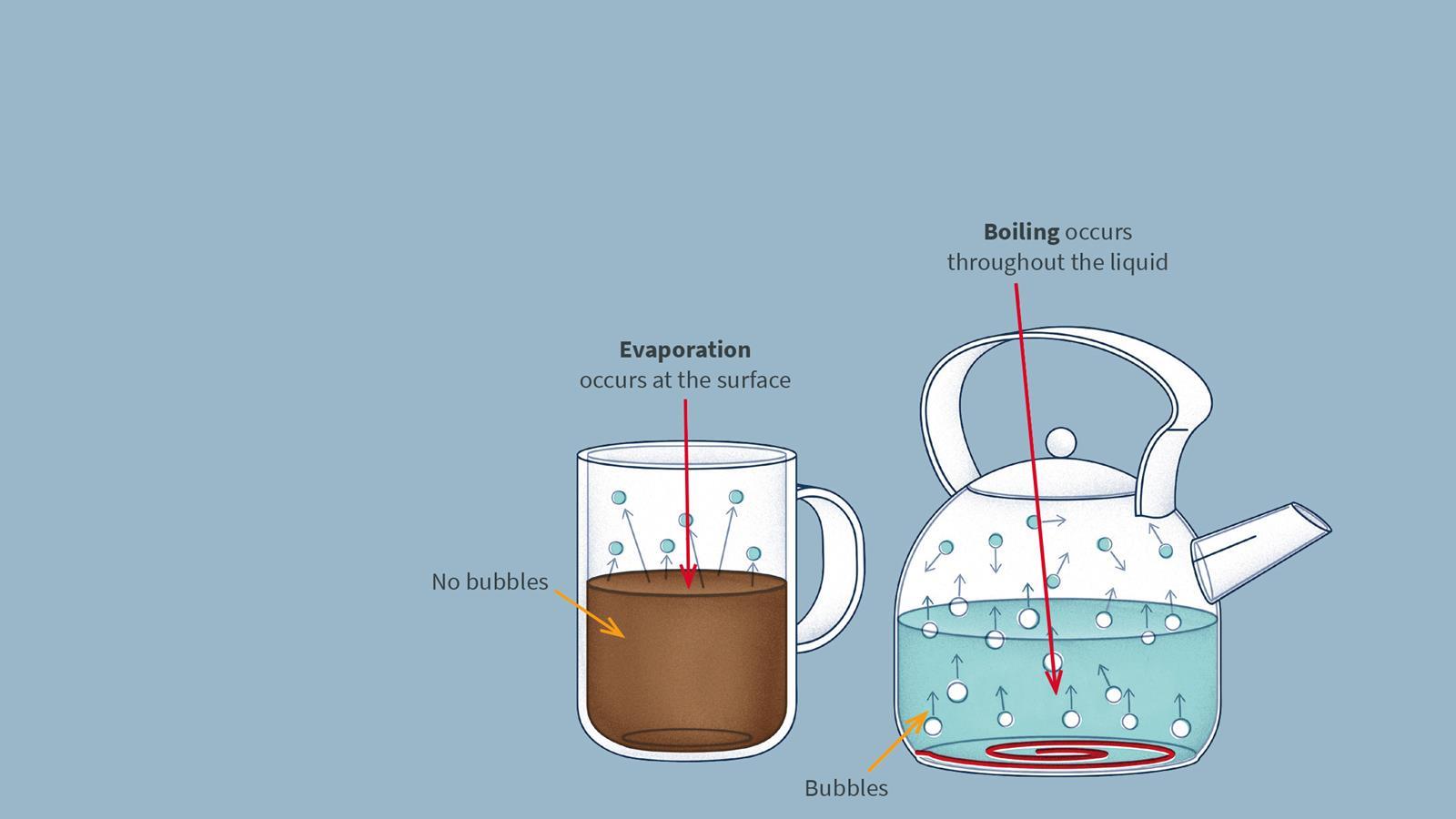
Evaporation is the process where a liquid changes to a gas. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles.
Evaporation explained
- Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point.
- The higher the temperature, the faster the evaporation.
- Some substances evaporate easily, some evaporate very little. It depends on the strength of the forces between particles.
Download this
This infographic is designed to be displayed as a poster in the classroom, although it can also be displayed on a projector or printed out as a handout.
Use the accompanying fact sheet and practical worksheet to explore evaporation and develop your students’ skills.
- Poster as pdf (A4 single pages or A3 one page)
- Fact sheet as MS Word or pdf
- Practical worksheet as MS Word or pdf.
View and download more infographics
In the bathroom
When you take a shower or bath, the hot water evaporates and produces lots of water vapour. You can see the water as it condenses on cooler surfaces like windows and mirrors.

Think about how condensation appears less in the summer when the room temperature is higher and you’re more likely to have the window open.
The ‘steam’ you can see emerging from a boiling kettle is actually tiny droplets of liquid water. Water vapour is invisible.
In a pandemic
Surface cleaning is important to prevent us picking up viruses from contaminated surfaces. When you spray door handles and other commonly touched surfaces with disinfectant, they don’t stay damp for long.
The layer of liquid evaporates quickly because it’s very thin and has a high surface area so more of the particles in the liquid are in contact with the air.
Hand hygiene has become more important during the pandemic. Next time you use an alcohol-based hand gel, rub the gel all over and give your hands a good shake – how do they feel?

In the kitchen
When a loaf of bread is baking, the inviting smell is due to evaporation of flavour compounds with complex names, such as 2-acetyl-1-pyrroline, that diffuse around the house.
And if you fancy some coffee with your toast, you’ll enjoy the aroma of 2-furylmethanethiol, among others.

All illustrations© Dan Bright
Teaching 11–14 learners?
Try these posters and activities on curriculum topics for younger students:
Downloads
Evaporation infographic poster A4 single pages
Presentation | PDF, Size 0.85 mbEvaporation infographic poster A3
Presentation | PDF, Size 0.82 mbEvaporation fact sheet
Editable handout | Word, Size 74.85 kbEvaporation fact sheet
Handout | PDF, Size 0.11 mbInvestigating evaporation
Handout | Word, Size 0.39 mbInvestigating evaporation
Editable handout | PDF, Size 0.3 mb






































No comments yet