Use this class practical to determine the relative atomic mass of magnesium using its reaction with hydrochloric acid
In this experiment, students react magnesium ribbon with dilute hydrochloric acid to produce hydrogen gas. They can then use the measured volume of hydrogen gas produced and the mass of magnesium to calculate the mass of magnesium required to produce one mole of hydrogen molecules. From this, students can deduce the relative atomic mass of magnesium.
This is a class experiment suitable for students who already have a reasonable understanding of the mole concept, and are at least beginning to use chemical equations to perform calculations.
Timing will depend on the adequacy of access to top-pan balances, and the skill with which students can use the balance to sufficient accuracy. Including the time taken by the teacher to demonstrate the procedure, and allowing an average of five minutes for each student to weigh their magnesium ribbon, a total of 45 minutes should be adequate for the class to obtain and record their results.
Equipment
Apparatus
- Eye protection
- Fine emery paper, a few cm, x2
- Burette, 50 cm3 (see note 1 below)
- Burette stand
- Funnel, small
- Beaker, 100 cm3
- Beaker, 250 cm3
- Access to a top-pan balance, accurate to +/– 0.001 g (see note 2)
- Access to room temperature and pressure measurements (see note 3)
Apparatus notes
- Ensure the burette taps are free from leakage, operate smoothly and are secure in their sockets. Refer to CLEAPSS Laboratory Handbook Section 10.10.1.
- If a balance weighing to 0.001 g is not available, reasonable results could be obtained by weighing a much longer (eg 30 cm) piece of magnesium ribbon beforehand on a balance weighing to 0.01 g, measuring its length and then cutting it accurately into 3 cm lengths. Using the mass and length of the long piece of magnesium, the average mass of a 3 cm length can be calculated with sufficient accuracy.
- If a barometer is not available in the laboratory, an up-to-date reading of atmospheric pressure will need to be obtained shortly before the lesson, eg from a local weather website. Similarly a measurement of room temperature is needed.
Chemicals
- Hydrochloric acid, 2 M, (IRRITANT), 25 cm3
- Magnesium ribbon, approximately 3–4 cm length
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Dilute hydrochloric acid, HCl(aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043. Provide the hydrochloric acid in small bottles or corked conical flasks, labelled, suitable for pouring the acid into the burette.
- Magnesium ribbon, Mg(s) – see CLEAPSS Hazcard HC059A. Clean the magnesium ribbon with emery paper to remove the grey oxide layer, so that it appears shiny and metallic. Cut the ribbon into lengths of 3–4 cm (which will yield around 30 cm3 of hydrogen when reacted). Do NOT leave in a place where pupils would have potentially unsupervised access.
Procedure
- Weigh accurately, to the nearest 0.001 g, a length of magnesium ribbon, approximately 3–4 cm long. The mass should lie between 0.020 and 0.040 g.
- Ensure the burette tap is closed. Use a small funnel to pour 25 cm3 of dilute hydrochloric acid into the burette, followed carefully by 25 cm3 of water. Try to avoid mixing of the two liquids as far as possible. Accurate volume measurements are not needed. This should leave a space of at least 5 cm between the liquid and the top of the burette.
- Carefully push the magnesium ribbon into the open end of the burette, pushing the strip in the middle so that the springiness of the strip holds it in place against the glass. Do not allow it to contact the liquid at this stage.
- Add about 50 cm3 of water to a 250 cm3 beaker.
- Rest the top of the burette gently on the lip of the beaker, then quickly turn the burette upside-down and lower the end beneath the water in the beaker. If this is done quickly and carefully (the teacher may wish to demonstrate this first), little or no liquid will be lost. Clamp the burette vertically in this upside-down position.
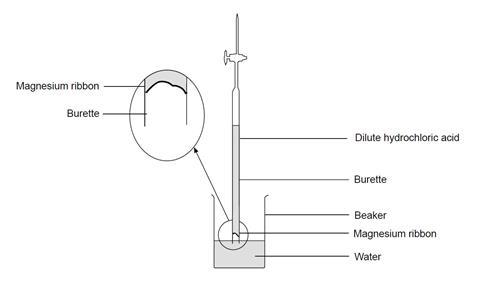
- Without delay, check that the liquid level in the burette is on the scale – if it is not, open the tap momentarily to allow the level to drop on to the scale.
- Take the burette reading of the liquid level. (Note: the scale is also now upside-down!)
- As the acid diffuses downwards, the magnesium begins to react. Allow the metal to react completely.
- Once the liquid level has ceased to change, and no more gas bubbles are being formed, take the final burette reading, and record the result.
Teaching notes
It is advisable to demonstrate the procedure beforehand. The inversion is not difficult, and it is not necessary to put a finger over the open end. Students need to be warned not to fold the magnesium ribbon, but to push it into the burette so that it is retained under its own tension.
A balance reading to only +/– 0.01 g does not have sufficient accuracy for the procedure used in this experiment, where the maximum possible volume of hydrogen that can be collected is only 0.002 mol, which would be produced by 0.048 g of magnesium.
The teacher may wish to collect results from the class on a spreadsheet to enable a discussion about their reliability.
The resulting calculation can be performed at various levels. The class do need to be able to understand the equation:
Mg + 2HCl → MgCl2 + H2
Students also need be able to use it to recognise that 1 mole of magnesium will yield 1 mole of hydrogen molecules. From the results, the least required of students would be to perform a proportionality calculation to determine the mass of magnesium that would have yielded 24 000 cm3 of hydrogen.
More able students, with an understanding of the ideal gas equation, should be asked to convert the volume of gas collected under known conditions in the experiment to standard temperature and pressure, and then determine the mass of magnesium that would have yielded 24 000 cm3 of hydrogen.
Further information
Most websites deal with instrumental procedures for determining atomic masses, such as mass spectrometry. Many of these are concerned with levels of detail far beyond what is appropriate even for more advanced students. However, Chemguide offers a fairly detailed treatment of mass spectrometry for determining atomic masses suitable for advanced students.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















2 readers' comments