Some metals are more reactive than others. Students will investigate competition reactions of metals and determine a reactivity series of the four metals used
In this experiment, a strip of metal is added to a solution of a compound of another metal. A more reactive metal displaces (pushes out) a less reactive metal from its compound. There are many ways of carrying out this series of reactions. The one described here uses a spotting tile but the same procedure could be adapted for use with test tubes. The advantages of the spotting tile method include:
- very small quantities of chemicals are used.
- the whole set of experiments is displayed together, making comparison easier.
- clearing-up afterwards is simple and avoids metal deposits being left in sinks.
Video support and linked resources
A version of this experiment is included in our Reactivity of metals video (from 10:35), along with supporting resources, including illustrated technician notes, integrated instructions, worksheets, a structure strip and more.
Careful thought needs to be given to distribution of the chemicals to the class. Solutions could be distributed in test tubes, or in small bottles fitted with droppers for sharing between several pairs of students. Metals could be issued in sets. The teacher should keep control of the magnesium ribbon, dispensing short lengths when required.
There should be no flames alight so that students are not tempted to burn pieces of magnesium and the teacher should be alert to the possibility of pieces of magnesium being removed from the laboratory.
The experiment should take about 30 minutes.
Equipment
Apparatus
- Eye protection
- Spotting tile, with at least 16 depressions (or two smaller tiles)
- Dropping (teat) pipette
- Beaker (100 cm3)
- Felt tip pen or other means of labelling
Chemicals
Access to about 5 cm3 each of the following 0.1 M metal salt solutions (note 1):
- Copper(II) sulfate (or nitrate(V))
- Lead(II) nitrate (TOXIC, DANGEROUS FOR THE ENVIRONMENT)
- Magnesium sulfate (or nitrate(V))
- Zinc sulfate (or nitrate(V))
- Five samples, approximately 1 cm lengths or squares, of each the following metals (note 2):
- Copper foil
- Lead foil (TOXIC, DANGEROUS FOR THE ENVIRONMENT)
- Magnesium ribbon
- Zinc foil
Chemical notes
- Solutions may be dispensed in 5 cm3 beakers to each pair of students or in small bottles fitted with droppers to groups of students.
- Metals should be approximately 1 cm lengths or squares of ribbon or foil cleaned with emery paper and as similar in size as possible.
Health, safety and technical notes
- Read our standard health and safety guidance
- Copper(II) sulfate solution, CuSO4(aq) – see CLEAPSS Hazcard HC027c.
- Lead nitrate(V) solution, Pb(NO3)2(aq), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC057a.
- Magnesium sulfate solution, MgSO4(aq) – see CLEAPSS Hazcard HC059b.
- Zinc sulfate solution, ZnSO4(aq) – see CLEAPSS Hazcard HC108b.
- Copper foil, Cu(s) – see CLEAPSS Hazcard HC026.
- Magnesium ribbon, Mg(s) – see CLEAPSS Hazcard HC059a. Do NOT leave in a place where pupils would have potentially unsupervised access.
- Zinc foil, Zn(s) – see CLEAPSS Hazcard HC017.
- Lead foil, Pb(s), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC056.
Procedure
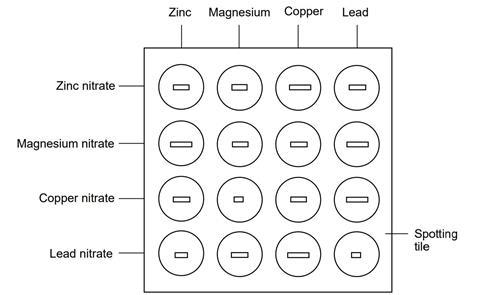
- Using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. Label this row with the name of the solution. Rinse the pipette well with water afterwards.
- Do this for each metal ion solution in turn, rinsing the pipette when you change solution.
- Put a piece of each metal in each of the solutions, using the illustration as a guide.
- Over the next few minutes observe which mixtures have reacted and which have not.
Teaching notes
Remind the class that they are looking for cases where one metal displaces another. Some of the solutions are slightly acidic so that bubbles of hydrogen are sometimes seen. Explain that this does not count as displacement of one metal by another.
It might be best to get the class to tell you what they think the order of reactivity is while they still have the evidence in front of them, so that apparent discrepancies can be resolved.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet