Create a small explosion in this demonstration by electrolysing water to produce bubbles of hydrogen and oxygen
In this experiment, students observe as water is electrolysed using sodium sulfate solution with a little universal indicator. The electrolysis produces the gases hydrogen and oxygen, which are allowed to mix and used to blow soap bubbles. When these bubbles are exposed to a Bunsen flame, students can witness the explosive recombination of hydrogen and oxygen, giving a loud ‘crack’.
This experiment works well as a class demonstration, and involves an impressive explosion between hydrogen and oxygen. This goes down well with students.
The demonstration takes about 10 minutes.
Equipment
Apparatus
- Eye protection
- Low voltage DC power pack, capable of supplying a current of at least 1 A at 12 V
- Connecting leads and crocodile clips
- Ammeter, 0–1 A
- Pieces of platinum wire, 10 cm length, x2 (see note 1 below)
- Clear glass jar, about 400 cm3 (see note 2)
- One-holed rubber bung to fit the jar
- Short length of glass tube, 6 mm diameter
- Length of flexible rubber, or silicone plastic tubing
- Beaker, 250 cm3
- Bunsen burner
- Spatula with spoon-shaped end (see note 3)
Apparatus notes
- It is best to use platinum for the positive electrode (anode), but the negative electrode (cathode) can be replaced by either nichrome or copper wire if preferred. Nickel could be used for both electrodes, provided you don’t run it for too long causing enough nickel to dissolve and colour the solution.
- The clear glass jar can be of the type normally used to store solids.
- If this type of spatula is unavailable use a long teaspoon – eg a metal spoon used for sundaes.
Chemicals
- Hydrated sodium sulfate, 10 g
- Washing up liquid, a few drops
- Universal Indicator solution (FLAMMABLE), a few drops
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Hydrated sodium sulfate, Na2SO4.10H2O(s) – see CLEAPSS Hazcard HC098B.
- Hydrogen gas, H2(g), (EXTREMELY FLAMMABLE) – see CLEAPSS Hazcard HC048.
- Oxygen gas, O2(g), (OXIDISING) – see CLEAPSS Hazcard HC069.
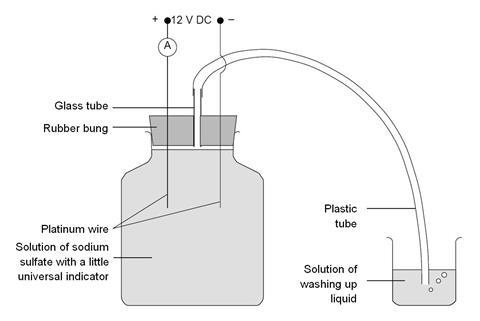
Procedure
Before the demonstration
- Set up the apparatus as shown in the diagram above. The electrodes can be inserted through the rubber bung by making extra holes with a piece of stiff wire or a straightened paperclip. First heat this to red heat in a blue Bunsen flame.
- Check that the apparatus is gas tight, and seal the wires with Vaseline, Blu-tac®, or silicone bath sealant if necessary.
- Make a solution of about 10 g of hydrated sodium sulfate in 500 cm3 of water.
- Half-fill the beaker with water, add a few drops of washing up liquid, and stir the mixture gently. Do not place the plastic tube in the beaker at this stage.
- Light a Bunsen burner, but keep it at least 1 m away from the electrolysis experiment.
The demonstration
Note
Eye protection must be worn throughout this demonstration because of the hydrogen produced (EXTREMELY FLAMMABLE).
- Fill the jar with the sodium sulfate solution leaving only enough air space to fit the bung. Add a few drops of universal indicator solution so that a green colour is clearly visible.
- Fit the bung and connect the electrodes to the 12 V power pack, incorporating an ammeter in the circuit.
- Switch on the power pack and adjust to give a current of about 1 A. The indicator turns blue around the cathode and yellow or orange around the anode. Bubbles of hydrogen are formed at the cathode and oxygen at the anode. It is worth pointing out that the volume of hydrogen formed is twice that of the oxygen produced.
- Observe these changes for a minute or so, to allow air in the delivery tube to be displaced by the mixture of hydrogen and oxygen. Now place the end of the tubing in the beaker of water containing the washing up liquid. Bubbles form and collect at the surface of the liquid.
- Scoop up some of the bubbles in the spatula and hold them in the Bunsen flame. They explode with an impressively loud sharp ‘crack’. If the bubbles do not explode, wait a little longer for the gas mixture to displace air from the tubing. (IMPORTANT: Under no circumstances try to ignite bubbles at the end of the tubing.)
Teaching notes
A white background behind the apparatus will allow the students to clearly see the colour changes in the sodium sulfate solution.
Some students may need the following explanation:
Water contains hydrogen and oxygen and electrical energy is causing water to split into these elements. The formula of water is H2O so you expect twice the volume of hydrogen to form as oxygen:
2H2O(l) → 2H2(g) + O2(g)
The explosion is caused by the energy released when the gases recombine to form water.
More able students can be given the following explanation.
Water, although covalent, ionises very slightly forming H+ ions and OH- ions:
H2O(l) ⇔ H+(aq) + OH-(aq)
However, there are insufficient of these ions to allow a reasonable current to flow, so an electrolyte – sodium sulfate – is added to increase the conductivity of the liquid. The sodium and sulfate ions are not discharged at the electrodes, but can be thought of as ‘spectator’ ions.
At the cathode water molecules are stripped of their hydrogen ions, which are then discharged, releasing hydrogen molecules and hydroxide ions. This is why the Universal indicator turns blue in this region:
2H2O(l) + 2e– → H2(g) + 2OH–(aq)
Water molecules at the anode yield up their hydroxide ions, which are discharged as oxygen, while releasing the remaining hydrogen ions into solution. This is why the Universal indicator turns yellow or orange here:
2H2O(l) → O2(g) + 4H+(aq) + 4e–
In a given time, as many electrons are supplied by the cathode as are received by the anode. Therefore it follows that two moles of hydrogen form (2H2) for every one mole of oxygen (1O2). As the number of moles of gas is proportional to its volume, this leads to the generation of two volumes of hydrogen for every one volume of oxygen.
It should be emphasised that, while electrical energy is being used to decompose the water into its component gases (an endothermic change), the explosion of these two gases represents the evolution of energy (an exothermic reaction). Also, the second reaction is the inverse of the first.
If a Hoffman voltameter, with platinum electrodes, is set up to do the electrolysis, the volumes of gas produced can be precisely measured, confirming the 2:1 volume ratio. If a Hoffman voltameter with carbon electrodes is used, the oxygen is partially converted to CO2 which partially dissolves.
It is worth discussing with students the feasibility of producing hydrogen on a large scale from water and using this gas as a perfect non-polluting fuel. This would be an alternative to fuels based on petrochemicals. Much research is being done to find a way of splitting water which avoids using fossil fuels in the process.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet