Displacement reactions as examples of redox reactions
This resource accompanies the Eduction in Chemistry article Any old iron.
Researchers at Eindhoven Technical University (TU/e) in the Netherlands are investigating the redox reaction between iron and oxygen to form iron oxide as a way of storing renewable energy. This reaction is an example of a redox reaction because as the iron is oxidised the oxygen is simultaneously reduced.
This is easier to see if we show the charges on the ions in iron oxide.
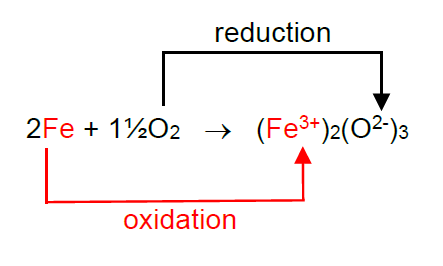
The Fe atom has lost three electrons to become a 3+ ion and so has been oxidised.
We show this in a half equation in which an electron is represented by e-.
Fe – 3e- → Fe3+
Each oxygen atom has gained two electrons to become a 2- ion and so has been reduced.
½ O2 + 2e- → O2-
A displacement reaction is another example of a redox reaction. In this task learners will look at displacement reactions and identify the oxidation and reduction processes occurring.
For each of the displacement reactions in the downloadable worksheet learners will need to:
- explain why the displacement reaction occurs.
- identify the metal that is oxidised and write a half equation for the oxidation process.
- identify the metal that is reduced and write a half equation for the reduction process.
In the two final examples learners will also need to write the symbol equation for the displacement reaction.
Remember, if a metal is a transition metal (or has a different charge to the group it’s in) the charge on its ion is shown in Roman numerals after the metal in the compound’s name, eg iron(III) chloride contains iron as an Fe3+ ion and has the formula Fe3+(Cl-)3.
More resources
- Find out about another alternative to the standard battery for energy storage in A step closer to post-lithium energy storage.
- Give students the opportunity to see the transfer of energy during the reaction of iron and oxygen while at the same time illustrating the overall increase in mass produced with the demonstration The combustion of iron wool.
- For guidance on how to introduce displacement reactions for the 11–14 age group, read Displacement reactions.
- Share this job profile of a PhD researcher who investigates new electrode materials for lithium-ion batteries.
Downloads
A fiery future questions
Editable handout | Word, Size 78.25 kbA fiery future questions
Handout | PDF, Size 0.12 mbA fiery future answers
Editable handout | Word, Size 68.5 kbA fiery future answers
Handout | PDF, Size 0.14 mb


















No comments yet