Synthesise and recrystallize a sample of methyl 3-nitrobenzoate.
Class practical
Nitration is the substitution of an NO2 group for one of the hydrogen atoms on a benzene ring.
In this experiment the students nitrate methyl benzoate. The reaction is regioselective and produces predominantly methyl 3-nitrobenzoate.
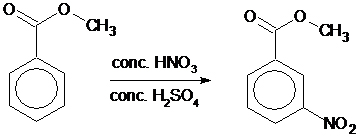
Lesson organisation
To synthesise and recrystallize a sample of methyl 3-nitrobenzoate will take about 1½ h. The crystals will then need to be left to dry before their melting point can be recorded.
|
Apparatus |
Chemicals |
|
conical flasks (1 × 50 cm3 and 1 × 100 cm3) balance spatula 10 cm3 measuring cylinders × 2 glass dropping pipette ice-water bath glass rod test tube thermometer (0–100 ° C) Buchner funnel apparatus melting point apparatus |
methyl benzoate (MODERATE HAZARD – harmful if swallowed) concentrated sulfuric acid (CORROSIVE – causes severe skin burns and eye damage) concentrated nitric acid (CORROSIVE AND OXIDISING – causes severe skin burns and eye damage. May intensify fire) methyl 3-nitrobenzoate (no known hazards) ethanol (FLAMMABLE – highly flammable liquid and vapour). IDA (FLAMMABLE – highly flammable liquid and vapour; MODERATE HAZARD – harmful if swallowed, may cause damage to organs) can be used instead. distilled water Refer to Health & Safety and Technical notes section below for additional information. |
Health & Safety and Technical notes
Read our standard health & safety guidance
Hand protection and goggles must be worn
Concentrated nitric acid and concentrated sulfuric acid are both highly corrosive. Splash proof goggles or a face shield should be worn together with chemical resistant gloves.
The nitrating mixture must be made in situ as required and kept cool throughout.
The recrystallization is carried out from a mixture of water and ethanol. This should be carried out on an electric hot plate to avoid naked flames.
Procedure
Preparation of methyl 3-nitrobenzoate
a Weigh 2.0 g of methyl benzoate into a dry 50 cm3 conical flask.
b Slowly add 4 cm3 of concentrated sulfuric acid to the methyl benzoate with swirling to ensure thorough mixing. Cool this mixture by partially immersing the flask in an ice-water bath.
c Carefully transfer 1.5 cm3 of concentrated nitric acid into a dry test tube. Cool the nitric acid by partially immersing it in an ice-water bath before slowly adding, with swirling, 1.5 cm3 of concentrated sulfuric acid. Ensure thorough mixing. Allow this mixture to cool. This is the nitrating mixture.
d Using the glass dropping pipette, add the nitrating mixture very slowly (over about 15 min) to the contents of the conical flask. Stir the reaction mixture as the addition is made. During the addition keep the temperature of the reaction mixture below 6 °C. NOTE: The nitrating mixture is very corrosive. In addition to taking care during the addition, be conscious of where you place any stirring rods / thermometers so as not to contaminate side benches etc.
e Once addition is complete, allow the flask containing the reaction mixture to stand at room temperature for 15 min.
f Carefully pour the reaction mixture onto a small amount (approx. 20 g) of crushed ice held in a beaker. Stir the crushed ice throughout. Solid methyl 3-nitrobenzoate will form.
g Allow the ice to melt and filter under suction. Wash the crude product with a little ice-cold water.
Purification and analysis
Methyl 3-nitrobenzoate is insoluble in water but soluble in hot ethanol. Therefore the crude material can be purified by recrystallization from a water/ethanol mixture.
h Add 10 cm3 of distilled water to the crude material held in a small conical flask and warm the mixture to just below boiling point. The methyl 3-nitrobenzoate will melt at this temperature but you will be able to see an ‘oily’ substance within the water.
i Slowly add hot ethanol 1 cm3 at a time until the ‘oily’ substance just dissolves.
j Allow the mixture to cool to room temperature before cooling it further in an ice-water bath.
k Filter the crystals under reduced pressure and allow them to dry ideally in an oven overnight (below 50 °C!).
l Record the melting point of your sample of methyl 3-nitrobenzoate.
Teaching notes
The nitration of methyl benzoate is an example of electrophilic substitution.
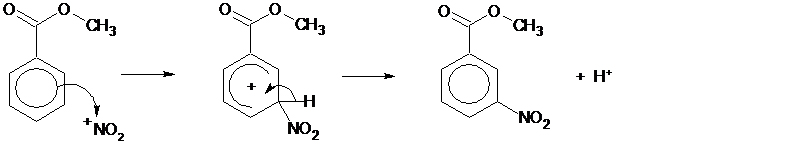
The carbonyl group withdraws electron density from the ring deactivating it towards electrophilic substitution. However the 3-position is less deactivated towards nitration than the other positions owing to the relative stability of the different intermediates. Therefore the reaction is regioselective for nitration at the 3-position.
More resources
Add context and inspire your learners with our short career videos showing how chemistry is making a difference.
Health & Safety checked, September 2016
Page last updated October 2016
Downloads
Nitration of methyl benzoate - student sheet
Experiment | PDF, Size 0.22 mb
Additional information
The Royal Society of Chemistry would like to thank former RSC School Teacher Fellow Catherine Smith (Teacher, Hinckley Academy and John Cleveland Sixth Form Centre) for trialling and writing up this practical, and David Armstead, on whose work it is based.
Image © Shutterstock.


















No comments yet