Prepare crystals of two soluble salts by reacting copper(II) oxide with dilute sulfuric acid, producing blue copper(II) sulfate
These two experiments should take no more than 30 minutes each to the point at which the solution produced has been filtered. If reagent solutions are provided in ready-measured quantities in small labelled bottles, and the solid powder reagents provided in roughly 1 g quantities in labelled specimen tubes, the experimental work can start immediately.
The experiments could be worked through in sequence, or allocated so that each group attempts only one preparation.
Most classes should be able to perform this experiment as a class experiment, but if there are real doubts about safe behaviour or adequate manipulative skills while solutions are being heated, or hot solutions being poured into the filter paper, then student-aided demonstrations of these may be more sensible.
Equipment
Apparatus
- Eye protection
- Beaker, 100 cm3
- Conical flask, 100 cm3
- Spatula
- Glass stirring rod, 15 cm
- Evaporating basin, 50–100 cm3 capacity
- Crystallising dish
- Filter funnel, 65 mm diameter
- Filter paper
- Bunsen burner
- Tripod
- Gauze
- Heat resistant mat
- Pipeclay triangle
- pH indicator paper
Chemicals
- Sulfuric acid, 0.5 M (IRRITANT), 20 cm3 (in a small bottle), x2
- One or more of the following, according to the lesson organisation:
- Copper(II) oxide (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), about 1 g (in a specimen tube)
- Magnesium carbonate, about 1 g (in a specimen tube)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection.
- Dilute sulfuric acid, H2SO4(aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book RB098.
- Copper(II) oxide, CuO(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC026.
- Magnesium carbonate, 3MgCO3.Mg(OH)2.3H2O(s) – see CLEAPSS Hazcard HC059b.
Procedure
Stage 1
Preparation 1: copper(II) sulfate
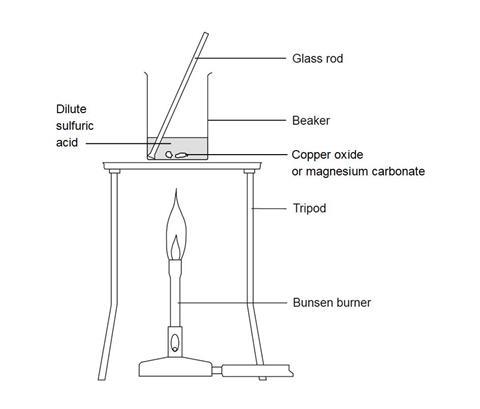
- Add 20 cm3 of 0.5 M sulfuric acid to the 100 cm3 beaker and heat carefully on the tripod with a gentle blue flame until nearly boiling. Be very careful not to knock the tripod while the beaker is supported by it.
- When hot enough, use a spatula to add small portions of copper(II) oxide to the beaker, stirring gently for up to half a minute after each addition. When adding the solid to the beaker, move carefully and slowly to avoid knocking the beaker over. You should not be sitting down.
- When all the solid has been added, continue to heat gently for one or two minutes to ensure the reaction is complete. If the mixture is a clear solution, add a little more copper(II) oxide and stir. To test that the reaction is complete check the solution pH with indicator paper – it should be neutral when it is ready. If not neutral continue adding copper(II) oxide and stir with heating. Once the solution is neutral turn off the Bunsen burner.
- Allow the beaker to cool slightly while you set up the next experiment.
Preparation 2: magnesium sulfate
- Add 20 cm3 of 0.5 M sulfuric acid to a clean 100 cm3 beaker. Use a spatula to add small portions of magnesium carbonate to the beaker, stirring gently for up to half a minute after each addition. (Do not heat the beaker at this stage when adding magnesium carbonate.)
- When all the solid has been added, heat the beaker gently for one to two minutes on a tripod over a small flame to ensure the reaction is complete and remove the heat. If the resulting mixture is a clear solution, add a little more magnesium carbonate and stir. To test that the reaction is complete check the solution pH with indicator paper – it should be neutral when it is ready. If not neutral continue adding magnesium carbonate until it is.
Stage 2 – repeat for each preparation separately
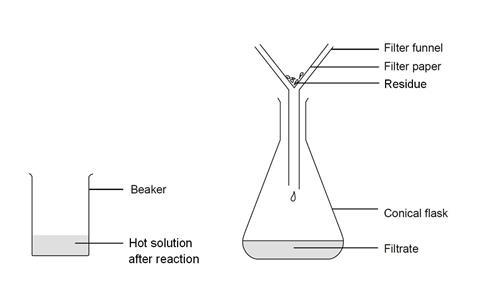
- Place the filter funnel in the neck of the conical flask.
- Fold the filter paper to fit the filter funnel, and put it in place.
- Make sure the beaker is cool enough to hold at the top, but the contents should still be hot.
- Swirl the contents gently to mix, and carefully pour into the filter paper in the funnel. Allow to filter through.
- A clear solution should collect in the flask. If the solution is not clear, and any solid remains in it, you will need to repeat the filtration.
Stage 3
- Pour the clear solution into an evaporating basin and place on a pipeclay triangle or gauze on the tripod.
- Heat the solution gently over a medium Bunsen flame so that water boils steadily. To avoid excessive fumes filling the lab ensure you do not boil dry the solution.
- When about half the water has boiled away, take a drop of the hot solution on the end of a glass rod and let it cool. If the drop crystallises on cooling, the solution is ready for the next stage; if it does not crystallise, keep boiling and repeat the testing until the drop does crystallise on cooling. (Do not boil the solution dry.)
Stage 4
- Pour the hot solution carefully into a crystallising dish.
- Set it aside to cool, with a label attached giving the name of the salt being prepared and the names of the group.
- Leave the crystallising dish in a warm place, safe from interference, until it has produced a good crop of crystals.
- If necessary, filter the solution, collect the crystals from the filter paper onto a paper towel and allow to dry.
Teaching notes
There may problems associated with younger students heating beakers perched on tripods, and with lifting hot glassware off a hot tripod after heating. Students should not be sitting down on lab stools whilst whilst handling hot solutions. Using tongs of suitable size is a good solution for lifting the hot beakers, but some schools may not have these. If there is any doubt about the safety of this step, you should lift each beaker down onto the heat resistant mat first before the students pour the contents into the funnel.
In stage 1 students should note any colour change in the solution (in preparation 1 from colourless to blue, at the same time as the black powder ‘disappears’; no colour change but fizzing in preparation 2).
In stages 2–4 of preparation 1, younger students are expected to use their previous experience of blue solutions/crystals to recognise the familiar colour of copper sulfate.
No such visual help is available in preparation 2. Older students already familiar with acid/base reactions should use that understanding to predict the identities of the compounds formed.
The symbolic equations for the reactions are only relevant for older students:
Preparation 1: CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l)
Preparation 2: MgCO3(s) + H2SO4(aq) → MgSO4(aq) + CO2(g) + H2O(l)
(‘Magnesium carbonate’ is unlikely to be pure MgCO3, which only occurs as such in certain minerals, but a basic salt also containing Mg(OH)2.)
In preparation 2, the product, magnesium sulfate, is commonly known as Epsom salts, though general familiarity with this substance and its uses is decreasing. However, it can help to broaden the interest in what many students find to be obscure substances with funny names if they do some research into their wider occurrence and uses.
Student questions
Stage 1
-
What change(s) do you see as you add the solid?
Stage 2
- What do you think is present in the clear filtered solution? Give a reason for your answer.
Stage 4
- What substance do you think these crystals are? Give a reason for your answer.
- Write word and symbol equations for the reaction you have done.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet