A negative test with a positive outcome
This activity is in two parts – in the first, students make observations while carrying out the tests for various negative ions. In the second, they use their observations to help them identify the negative ions present in a number of unknown solutions.
To make the second part of the exercise more challenging, tests for positive ions could be introduced and students could be asked to identify both the positive and negative ions present in a solution.
Equipment required
The exact concentrations of the test solutions are not important.
Use approximately 0.1–0.5 mol dm–3 for salt solutions and 0.5–1.0 mol dm–3 for acid solutions, except for nitric acid, which is corrosive at such concentrations (use 0.4 mol dm–3 instead).
- Test-tubes
- Dropping pipettes (these can be used just for the carbon dioxide testing or also for dispensing solutions; if the latter, far more pipettes will be required)
- Nitric acid 0.4 mol dm–3 (Irritant)
- Silver nitrate solution 0.1 mol dm–3
- Barium chloride solution 0.1 mol dm–3 (Harmful)
- Hydrochloric acid
- Aluminium powder (Highly flammable)
- Sodium hydroxide solution less than 0.5 mol dm–3 (Irritant)
- Limewater
- Red litmus paper
- Ammonia solution 0.4 mol dm–3
For the initial observations
- Sodium or potassium chloride solution
- Sodium or potassium bromide solution
- Sodium or potassium iodide solution
- Sulfate solution, eg sodium sulfate
- Carbonate solution, eg potassium carbonate
- Nitrate solution, eg potassium nitrate.
For testing unknowns
The number of unknowns required depends on the time available. It is a good idea to use at least four solutions to ensure students are challenged.
Label the solutions A, B, C etc and make sure you know which is which.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection.
- Barium chloride solid is toxic; the 0.1 mol dm–3 solution is harmful. See CLEAPSS Hazcard HC010a
- Wash your hands after use and warn students to do the same.
- Ammonia solution is an irritant when concentrated but not at the concentrations used by students in this activity. However, it can give off ammonia vapour, which can irritate the eyes and lungs.
- Keep the lid on the bottle when not in use.
- Nitric acid is an irritant. See CLEAPSS Hazcard HC067
- Silver nitrate solution can stain skin and clothes. See CLEAPSS Hazcard HC087
Tests for negative ions – expected observations
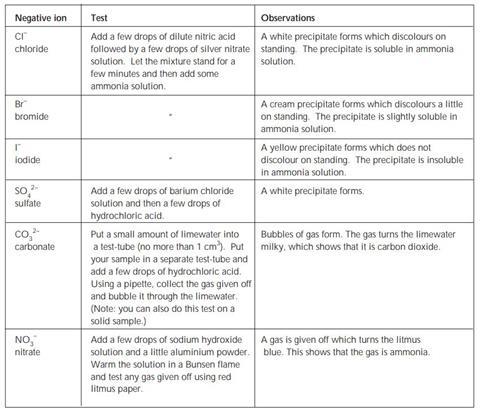
Testing for negative ions – making observations
This activity is in two parts. In the first part you observe the reactions of various negative ions and in the second you use those observations to identify unknown solutions.
Use the table Tests for negative ions to record your observations during each test. Use a clean test-tube each time or wash up thoroughly between tests using distilled or deionised water to avoid contamination. Use a small portion of the test solution each time (no more than 1 cm3).
Write balanced symbol equations for the reaction that occurs in each of the tests (except the test for a nitrate).
Downloads
Testing for negative ions
PDF, Size 0.27 mb
Additional information
This resource is a part of our Inspirational chemistry collection.


















No comments yet