Use these classroom tips and ideas to effectively teach your post-16 students about transition metal complexes

Why is blood red? Why, for some sea creatures, is blood blue? The answer lies in the fascinating and colourful topic of transition metal complexes. While the coordination of iron(II) is responsible for the red colour in haemoglobin, it is copper(II) that gives haemocyanin its blue colour. When deoxygenated, it is colourless; when oxygenated, it’s blue. Transition metal ions, surrounded by ligands coordinating to a central metal ion, have been used in medicine for decades – in applications such as anti-cancer treatments or as tracers providing diagnostic information.
D-orbitals, electrons and energy gaps can seem hugely abstract to students, so real-world contexts, and the wonderfully colourful chemistry of transition metal complexes – that they can actually see – offer a tangible route to learning the underlying chemistry.
What students need to know
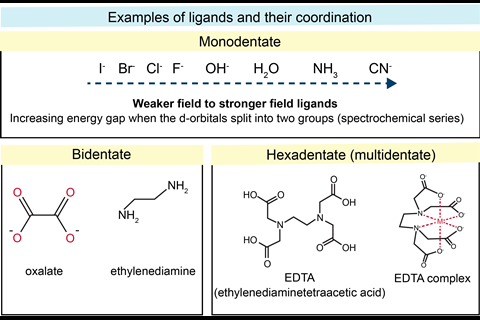
To understand transition metal ions and their complexes, students need to be secure in their grasp of detailed electronic arrangements and d-subshells. A transition metal complex consists of a central metal ion surrounded by ligands. A ligand is an ion or molecule with a lone pair of electrons to donate to form a coordinate (dative covalent) bond with a transition metal ion. The coordination number of the complex is the total number of bonds from the ligands to the central transition metal.
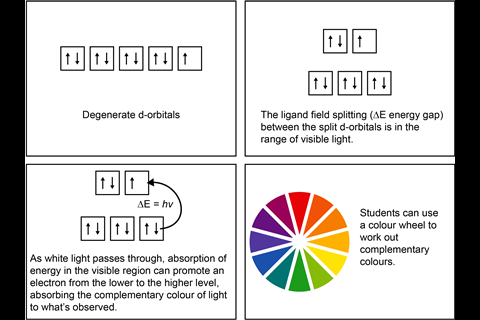
The d-orbitals are no longer degenerate in a complex of a transition metal due to the repulsions of orbitals on particular axes with approaching ligands and a change in energy.The colours of most transition metal complexes are due to d−d electron transitions absorbing the complementary colour of light to what’s observed. The colour depends on the transition metal, oxidation state, ligands and coordination number. A transition metal can exist in a range of oxidation states in its compounds, and can be readily inter-converted.
Common misconceptions
- The word complex can be misleading. Explain to your students that it doesn’t mean complicated in this context, and refers to the compound consisting of multiple parts.
- Electronic configurations with filling of d-orbitals, sub-shells and d−d transition of electrons can seem abstract to students.
- Learners can confuse absorption and emission. The absorption of light will show complementary colours, eg a solution appears violet in colour because the solution absorbs yellow light, which is the complementary colour to violet.
- Students can find it hard to fully explain why zinc compounds are colourless.
- They need to understand that both electrons in the coordinate (dative covalent) bond come from the same atom.
- Learners can also struggle to recognise a change in oxidation state.
- Many of the terms and vocabulary used to teach this topic will be new to students. They can find it hard to name complexes due to their length and pupils’ lack of familiarity with some of the parts.

Download this
Transition elements and complex compounds microscale experiment, for age range 16–18
Investigate transition metal chemistry and conduct a series of reactions to model sustainable practices.
Resources include:
Download this
Transition elements and complex compounds microscale experiment, for age range 16–18
Investigate the chemistry of the transition elements using sustainable practices with this microscale practical, from the Education in Chemistry website: rsc.li/3LrofVu
Ideas for your classroom

When introducing transition metal ion complexes, start with a simple, familiar example. A test tube of copper(II) sulfate in aqueous solution shows the well-known blue colour due to the Cu2+ ions complexing with water. Get students to draw this hexaaqua octahedral structure and write out the d-electrons and splitting. Add ammonia solution until the sample turns dark blue, as water ligands are replaced by ammonia, to show how a stronger field ligand affects the colour. Further outline the sequential replacement of ligands by looking at copper complexes. This can be used at a later stage to explore ligands, chelation and linking equilibria and stability constants.
Use microscale to give your students the opportunity to closely observe a selection of transition metal complexes, their colours, and the changes they undergo when adding other ligands and reagents. It also offers opportunities for discussion about the observed changes. Can your students identify evidence of complex formation? Is there a change in oxidation state? And why is there no colour with zinc?
Looking at vanadium complexes in more depth will prompt your students to consider variable oxidation states. Start from the yellow V(V) ions (VO2+) that are reduced by zinc in an acidic solution first to the blue V(IV), and so on, all the way to the purple V(II) ions. Then use potassium permanganate (another transition metal with a high oxidation state) to oxidise vanadium back through all these changes to +5. Get your students to think about electrode potentials and making predictions.
More resources
- Provide real-world applications of transition element complexes and link to the theory of molecular shapes and stereochemistry by looking at transition metals and anticancer drugs and cisplatin and drug design.
- Link your lessons on d-block elements and catalysts to careers with museum scientist Lucia’s video job profile and find more chemistry roles that inspire change.
- Play transition metal games to develop your learners’ understanding, vocabulary and thinking skills.
- Give your 16–18 year-old learners valuable retrieval practice and revision using the Starter for 10: transition metal chemistry.
More resources
- Provide real-world applications of transition element complexes and link to the theory of molecular shapes and stereochemistry by looking at transition metals and anticancer drugs (rsc.li/48j4IAg) and cisplatin and drug design (rsc.li/46eyGU7).
- Link lessons on d-block elements and catalysts to careers, with a museum scientist’s video job profile: rsc.li/46hvV4s
- Play transition metal games to develop learners’ understanding, vocabulary and thinking skills: rsc.li/48mBhgW
- Plan in retrieval practice and revision exercises for your your 16–18 learners: rsc.li/48dEPC5
Many transition metal complexes make good catalysts due to the availability of their d-orbitals, and ability to change oxidation states by donating or gaining electrons from reactants. The visible activated complex practical activity is well known, showing a reaction catalysed by cobalt(II) chloride, where the pink colour changes to green (activated complex), and back to pink. Students (if they are quick) can transfer a small sample of the green cobalt(III) species to a test tube on ice to trap it. They can then link oxidation states to observations, for instance where hydrogen peroxide initially oxidises the Co2+ ions (pink) to Co3+ ions (green). The tartrate ions present form a complex with cobalt(III). These tartrate ions are then oxidised as the green Co(III) reduces to the characteristically pink Co(II). Use this example to reinforce that as the oxidation state of the metal increases, the splitting of the d-orbitals also increases.
Many transition metal complexes make good catalysts due to the availability of their d-orbitals, and ability to change oxidation states by donating or gaining electrons from reactants. The visible activated complex practical activity (rsc.li/3PpWcqm) is well known, showing a reaction catalysed by cobalt(II) chloride where the pink colour changes to green (activated complex), and back to pink. Students (if they are quick) can transfer a small sample of the green cobalt(III) species to a test tube on ice to trap it. They can then link oxidation states to observations, for instance where hydrogen peroxide initially oxidises the Co2+ ions (pink) to Co3+ ions (green). The tartrate ions present form a complex with cobalt(III). These tartrate ions are then oxidised as the green Co(III) reduces to the characteristically pink Co(II). Use this example to reinforce that as the oxidation state of the metal increases, the splitting of the d-orbitals also increases.
Assessing student understanding
Throughout this topic, give students plenty of opportunity to practise the various key aspects, such as drawing d-electrons, showing what happens to electrons when energy is absorbed, and drawing complex ions showing the coordination of ligands with 3D geometry. If they answer on mini whiteboards, you can quickly view answers to assess who has understood or needs more help. Follow up with microscale practical activities so students can make links to concrete examples of selected metal ions, ligands and colours.
Take-home points
- Try using microscale practicals to promote thinking. Encourage students to draw the structures and name them if appropriate. Link exchanges of ligand and different metal ion or oxidation state with the corresponding changes in colour absorbed and complementary colour observed.
- With coordinate (dative) bonding, make it clear that both electrons come from the ligand as either negative charge or lone pair.
- Link to useful applications of transition metal ions, particularly catalysts, relating observations such as colour change to a change in oxidation state.
- Provide lots of opportunities to practise examples.
- Regularly make connections with other chemistry topics. Boost understanding by linking transition metal complexes and equilibria (ligand exchange and stability constants) oxidation and reduction reactions, electron donors and acceptors, within a complex and analysis with colorimetry.
This article was updated on 25 June 2024.
Article by Morag Easson, a chemistry teacher in Scotland and Royal Society of Chemistry trainer. Resource by by head of chemistry Nicola Kiernan









1 Reader's comment