A question and answer sheet that tests learner’s knowledge of transition metal chemistry
The topics covered in this Starter for ten activity are: transition metals recap, transition metal complexes, colours of complex ions, colorimetry, redox titrations, redox chemistry of transition metals, and transition metals as catalysts.
Example questions
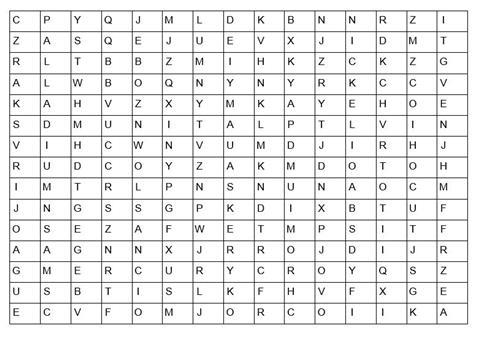
Find in the word search above, the answers to each of the questions below to gain the mark(s);
- The catalyst in the Haber process used to make ammonia
- 3 Common catalysts found in the catalytic converter
- The aqueous solution of this metal sulphate is blue
- This metal has a melting point of -38.3 ºC
- These two transition metals are found in the smart alloy, nitinol.
- These two metals when in oxidation states +6 and +7 respectively and combined with oxygen are common oxidising agents used in organic chemistry.
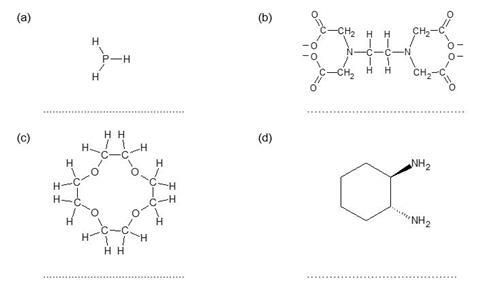
A transition metal complex consists of a central metal ion surrounded by ligands. A ligand is an ion or molecule with a lone pair of electrons that forms a coordinate bond with a transition metal ion.
For each of the ligands below, mark on any lone pairs which are able to form coordinate bonds, and identify the ligand as unidentate (can form one coordinate bond), bidentate (can form two coordinate bonds) or multidentate (can form three or more coordinate bonds).
Notes
A full version of this question and answer sheet is available from the ‘downloads’ section below. An editable version is also available.
Downloads
Transition metals
PDF, Size 0.62 mbTransition metals - editable
Word, Size 0.53 mb
Starters for 10: Advanced level 2 (16–18)

This chapter in our Starters for ten series covers: kinetics, equilibria, acids and bases, carbonyl chemistry, aromatic chemistry, compounds with amine groups, polymers, structure determination, organic synthesis, thermodynamics, periodicity, redox equilibria, transition metal chemistry, and inorganics in aqueous solution.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
 Currently
reading
Currently
reading
Transition metal chemistry
- 15















































1 Reader's comment