Teaching strategies to help students get to grips with thermodynamics, including enthalpy changes and Hess’s law
It is crucial for students to understand thermodynamics so they can grasp how energy changes in chemical reactions affect the real world and products at their fingertips. Sometimes literally, with everyday applications in hand warmers and first aid instant cold packs. Concepts such as Hess’s law, enthalpy changes and the conservation of energy lay the foundations for advanced studies. By mastering these topics, students can better understand processes such as the synthesis of chemicals, the efficiency of industrial reactions and the environmental impact of chemical processes.
Use this guide as a framework to teach these concepts effectively, address common misconceptions and offer practical classroom activities. Although the guide is aimed at foundation concepts, I have included resources suited to more advanced topics, such as Born–Haber cycles and entropy.
What students need to know
To master thermodynamics in chemistry, students need a solid understanding of the following key concepts:
- Conservation of energy: energy cannot be created or destroyed, only transformed. This principle underpins enthalpy cycles and Hess’s law.
- Enthalpy as a state function: enthalpy depends only on the initial and final states of a system, not the path taken. This concept is crucial for understanding why Hess’s law works.
- Hess’s law: the enthalpy change of a reaction is the same regardless of the route taken, providing the start and end conditions are the same.
- Standard enthalpy changes of reaction (ΔH⦵r), formation (ΔH⦵f) and combustion (ΔH⦵c): we measure these under standard conditions (298 K, 100 kPa, 1 mol dm-3). When students understand these definitions, they can better construct accurate enthalpy cycles.
- Sign convention: the correct interpretation of exothermic (negative enthalpy change) and endothermic (positive enthalpy change) reactions is essential for accurate calculations.
Common misconceptions
Exam reports often highlight errors in constructing and interpreting enthalpy cycles, perhaps because students have an incomplete understanding of the definitions. In formation reactions one mole of a compound is created from its elements in their standard states, while in combustion reactions one mole of a substance is burned in oxygen. When students know the specifics of these reactions they can calculate enthalpy changes in a systematic way, construct enthalpy cycles and apply Hess’s law.
Students often struggle with the concept that energy conservation means the total energy change of a reaction is independent of the path taken. When students do not understand enthalpy as a state function it can lead to confusion when they apply Hess’s law.
Read the article How to teach energy and change at post-16 for more misconceptions related to these topics.
Read the article ‘How to teach energy and change at post-16’ for more misconceptions related to energy and change (rsc.li/3AfIXVp).
Ideas for your classroom
Provide clear, step-by-step instructions and scaffolded support when teaching enthalpy. Gradually reduce support as students become more confident, ensuring a solid understanding of each step before moving on.

Download this
Lesson plan with experiment, for age range 16–18
Introduce your students to Hess’s law and measure enthalpy changes using two simple practical activities.
Resources include:
Download this
Lesson plan with experiment, for age range 16–18
Introduce your students to Hess’s law and measure enthalpy changes with two simple practical activities.
Download the resource from the Education in Chemistry website: rsc.li/3WMkw9E
Explain that a Hess cycle (figure 1) is a diagram that shows alternative routes between reactants and products which you can use to calculate unknown enthalpy changes using known enthalpy changes. Being able to construct and analyse these cycles is a practical application of Hess’s law and is essential for solving many thermodynamic problems in chemistry.
Step-by-step guide to constructing a Hess cycle:
- Write the balanced chemical equation, including state symbols, for the reaction.
- Identify known enthalpy changes (e.g. formation or combustion).
- Draw the cycle, showing alternative routes from common reactants to common products.
- Use Hess’s law to set up equations relating the enthalpy changes around the cycle.
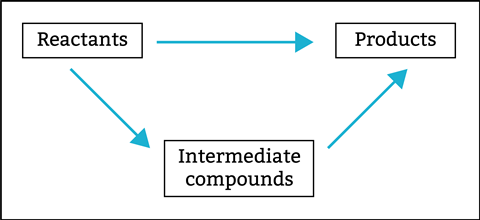
Look out for confusion over the signs (positive or negative) of enthalpy changes and make sure that the arrows are pointing in the correct direction. Encourage students to check if they need to multiply equations. You will find further support and suggestions for drawing Hess cycles in this CPD article.
Look out for confusion over the signs (positive or negative) of enthalpy changes and make sure that the arrows are pointing in the correct direction. Encourage students to check if they need to multiply equations. You will find further support and suggestions for drawing Hess cycles in this CPD article (rsc.li/3WBDy2o).
Use visual aids, interactive simulations and hands-on experiments to make abstract concepts tangible. Start with practical work to observe an example of Hess’s law in practice, followed by an instructed cycle and compare the values obtained. Use other examples to promote discussion and problem solving.
More resources
- Use practical videos as pre-laboratory preparation to reduce the cognitive load of practical work.
- Measuring enthalpy changes: teaching tips for your classroom provides further ideas for practical work, including a microscale method for measuring enthalpy changes of reactions in solution.
- Provide a real-life context to act as a memory hook for learners. Get students working with thermochemistry, intermolecular forces and free energy using the Contexts for thermodynamics infographic and the Runaway reactions resource.
- Show learners how chemistry is making a difference to the world around them with A Future in Chemistry job profiles.
Use the downloadable resource to introduce Hess’s law with a simple experiment. Get students to collect experimental data to measure the enthalpy change of hydration of copper(II) sulfate, which you cannot measure directly. When measuring enthalpy changes, students often forget that the thermometer measures the temperature of the surroundings. Remind students that an increase in thermometer reading during an experiment indicates an exothermic reaction, as the system releases energy to the surroundings.
More resources
- Use practical videos as pre-laboratory preparation to reduce the cognitive load of practical work: rsc.li/4dD2ThK
- Measuring enthalpy changes: teaching tips for your classroom provides further ideas for practical work, including a microscale method for measuring enthalpy changes of reactions in solution: rsc.li/3SM9yQk
- Provide a real-life context to act as a memory hook for learners. Get students working with thermochemistry, intermolecular forces and free energy using the Contexts for thermodynamics infographic (rsc.li/3SLWmv4) and the Runaway reactions resource: rsc.li/3WJpuXV
- Show learners how chemistry is making a difference to the world around them with A Future in Chemistry job profiles: rsc.li/3yCzwPp
Checking for understanding
Encourage regular practice with a variety of problems. Use formative assessments like Thermodynamics starters for 10 to identify and address difficulties.
Promote group work and discussions where students can explain concepts to each other, reinforcing their understanding.
Use infographics as a talking point to facilitate peer teaching by covering the text or an image and asking students to fill in the blank. For example, use Mastering the Born–Haber cycle when introducing the application of Hess’s law to the formation of ionic compounds.
Encourage regular practice with a variety of problems. Use formative assessments like ’Thermodynamics starters for 10’ to identify and address difficulties (rsc.li/3Aqe29K).
Promote group work and discussions where students can explain concepts to each other, reinforcing their understanding.
Use infographics as a talking point to facilitate peer teaching by covering the text or an image and asking students to fill in the blank. For example, use ’Mastering the Born–Haber cycle’ when introducing the application of Hess’s law to the formation of ionic compounds (rsc.li/3Axn8kf).
Take-home points
- Energy conservation: emphasise that energy cannot be created or destroyed.
- Enthalpy as a state function: highlight its importance in applying Hess’s law.
- Standard enthalpy changes: draw out Hess cycles to practise applying definitions.
- Connect concepts to real-world chemical processes to make them more relatable and to demonstrate their importance.
Article written by Naomi Hennah. Resource updated from the Assessment for learning collection
















No comments yet