Share this infographic with your students, download the poster for your classroom and get students working with thermochemistry, intermolecular forces and free energy
Chemical thermodynamics describes the change in enthalpy (∆H) and entropy (∆S) during a chemical reaction or change of state. The process will only be possible if the enthalpy and entropy changes combine to give a negative value for the change in free energy (∆G), where ∆G = ∆H – T∆S.
The formation of bonds is exothermic, which decreases the enthalpy because energy is transferred as heat to the surroundings. The stronger the bonds, the more energy is transferred as heat to the surroundings. Breaking bonds has the reverse effect and so is endothermic.
Entropy increases during processes that involve a disordering of the energy, such as the formation of gases.
Download this
This infographic is designed to be displayed as a poster in the classroom, although it could also be displayed on a projector or printed out as a handout. Use the accompanying fact sheet and activity to explore Hess’s law, intermolecular forces, entropy change and free energy.
- Poster as pdf (A4 single pages or A3 one page)
- Fact sheet as MS Word and pdf
- Teacher notes and worksheet for age range 16–18 as MS Word or pdf
- Answer sheet as MS Word or pdf
Getting back to the Moon
The NASA Artemis Moon mission aims to send a piloted spacecraft to the moon within the next decade. It will be sent on its way by the 98 m tall SLS rocket being built at Cape Canaveral.
- The four rocket engines of the main core stage will be powered by over 2500 m3 of supercooled liquified hydrogen and oxygen.
- The two additional booster rockets will be powered by a solid fuel similar to that used in fireworks.
- The engines will produce a total of 3.9 x 107 N of thrust, making it the largest rocket ever built.
Equation
Core stage fuel reaction: H2(l) + ½O2(l) → H2O(g) ∆H = – 241 kJmol-1
Thermodynamics
The large enthalpy of the combustion of hydrogen, combined with its very low density, allows the fuel and oxygen mixture to produce 13.4 MJkg-1 – the highest energy per kilogram of any chemical fuel. The energy is released when the very strong O–H bonds in water form, replacing the weaker H–H and O=O bonds in the reaction mixture.


Did you know? The velocity that the rocket can achieve depends on the energy released and on the velocity of the gas particles emitted. Reactions producing small, fast-moving molecules will create a more efficient fuel.
Did you know? Bicarbonate of soda relies on the presence of naturally occurring acids in the baking mixture. In recipes where these are not present in sufficient quantities, then baking powder, which also includes an acid, is used instead.
Did you know? Refrigerants such as difluoromethane have a global warming potential many times that of carbon dioxide, so it is important to ensure they never leak out.
Perfect biscuits and cakes
Baking often requires a raising agent to create bubbles in the mixture that will make it less dense when cooked. The most common raising agent is sodium hydrogen carbonate (sodium bicarbonate), which reacts with other dissolved acidic substances in the recipe. This produces carbon dioxide, giving a light airy texture.
Equation
NaHCO3(s) + H+(aq) → Na+(aq) + H2O(l) + CO2(g). ∆HO = +28 kJmol-1 ∆SO = +241 JK-1 mol-1
Thermodynamics
This reaction is endothermic because the bonds are stronger in the reactants than in the products, but it occurs because of the large increase in entropy that results from the formation of a gas. Therefore, the free energy change at room temperature (298 K) is negative overall: ∆G = +28 – (298 x 0.241) = –44 kJmol-1. At higher cooking temperatures, the free energy change becomes even more negative, helping to ensure that any remaining sodium hydrogen carbonate is fully reacted.
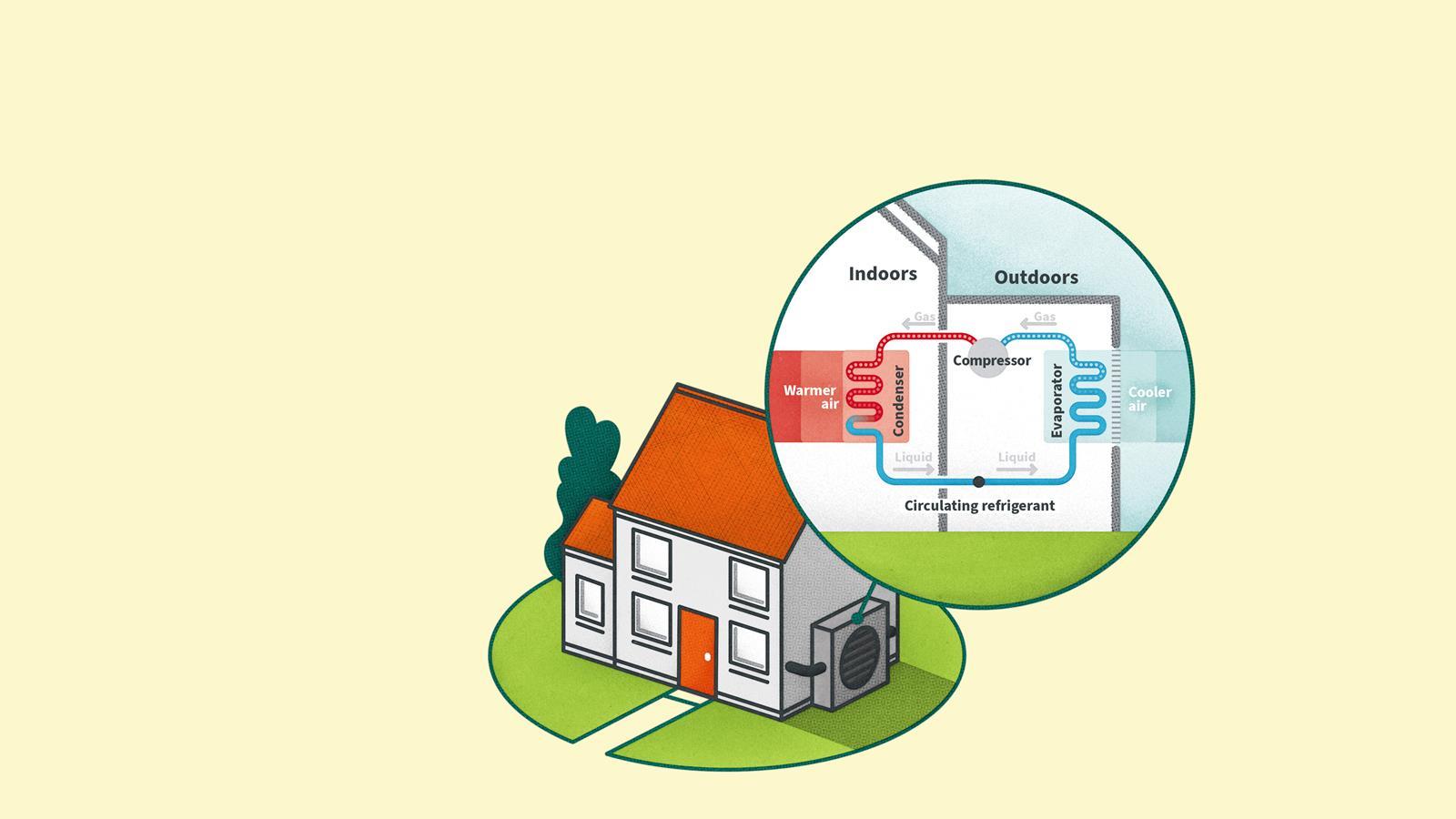
Warm homes for the future
One of the biggest challenges to reducing greenhouse gas emissions to zero by 2050 is replacing the gas and oil boilers that heat more than 90% of our homes. Heat pumps are currently one of the most promising carbon-neutral alternatives.
In a heat pump, a liquid (the refrigerant) is continually pumped between two coils – one at low pressure outside the house where it is cooler, and one at high pressure inside where it is warmer. An example of a refrigerant is difluoromethane.
Equation
Vaporisation of difluoromethane: CH2F2(l) ⇌ CH2F2(g) ∆HO vap = +20 kJmol-1
Thermodynamics
Le Chatelier’s Principle predicts that evaporation will be favoured by decreasing the pressure, as this direction increases the moles of gas.
- Outside: Pressure = 760 kPa, giving a boiling point of –2°C, so the refrigerant evaporates at temperatures above this, transferring energy to the gas as the intermolecular forces are overcome.
- Inside: Pressure = 3040 kPa, giving a boiling point of 49°C, so the refrigerant condenses at temperatures below this, transferring the energy as heat into the home.
Therefore, energy from the cooler air outside can be used to warm a house. If renewable energy is used to power the pump, then no greenhouse gases are emitted during the process.
Put this in context
Learn how a professor in chemistry at Swansea University leads research that mitigates climate change and has led to the production of energy-generating coatings for buildings.
Downloads
Thermodynamics infographic poster A4 single pages
Article | PDF, Size 0.3 mbThermodynamics infographic poster A3
Article | PDF, Size 0.29 mbThermodynamics fact sheet
Editable handout | Word, Size 83 kbThermodynamics fact sheet
Handout | PDF, Size 0.16 mbThermodynamics contexts teacher notes and worksheet
Editable handout | Word, Size 91.21 kbThermodynamics contexts teacher notes and worksheet
Handout | PDF, Size 0.2 mbThermodynamic contexts answers
Handout | PDF, Size 0.21 mbThermodynamic contexts answers
Editable handout | Word, Size 0.1 mb














No comments yet