Research suggests that students commonly focus on covalent and ionic bonding, and often fail to spot the importance of other types of bonding
This exercise is about the interactions between particles in different chemical systems (such as molecules, atoms, lattices). It has been found that different students have distinct ideas about how to label and describe the interactions that are found in chemical systems.
You are asked to identify and describe any interactions that are present between different parts of the chemical system in the diagram.
In each case you are asked whether you think that the interaction should be classed as an attraction, a force, bonding and/or a chemical bond. Please select ‘yes’, ‘no’, or ‘unsure’ (if you do not know).
You do not have to select one and only one ‘yes’ answer: you may select ‘no’ for all four options, or ‘yes’ for all four, or any combination of ‘yes’ and ‘no’ responses. You are also asked to label the type of interaction, if you think it has a special name, and to describe the interaction as best you can in your own words.
Questions
- The diagram represents a single atom of hydrogen.
Which, if any, of the following labels can be used to identify the interaction between the two parts of the system shown

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- The next diagram represents a single molecule of hydrogen.
Which, if any, of the following labels can be used to identify the interaction between the two parts of the system shown.

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- The diagram represents part of a layer in a sodium chloride lattice.
Which, if any, of the following labels can be used to identify the interaction between the two parts of the system shown.

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- (part b) The diagram represents part of a layer in a sodium chloride lattice.
Which, if any, of the following labels can be used to identify the interaction between the two parts of the system shown.

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- The diagram represents some water molecules in liquid water.
Which, if any, of the following labels can be used to identify the interactions between molecules in the liquid.

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- The diagram shows part of an aqueous solution of silver nitrate.
Which, if any, of the following labels can be used to identify the interactions between the ions and the molecules in the liquid:

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- The diagram represents a molecule of fluorine.
Which, if any, of the following labels can be used to identify the interactions which hold the molecule together?

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- The diagram represents iodine molecules in solid iodine.
Which, if any, of the following labels can be used to identify the interactions between the molecules?

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- The diagram represents the lattice arrangement in copper.
Which, if any, of the following labels can be used to identify the interactions holding the copper together?
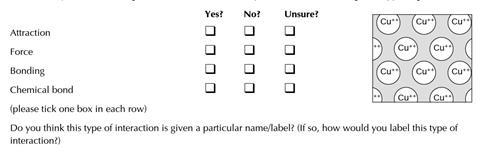
Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
- The diagram represents a dimer of aluminium chloride (AlCl3).
Which, if any, of the following labels can be used to identify the interactions between the two AlCl3 molecules?

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can
- The diagram represents part of the diamond structure of carbon.
Which, if any, of the following labels can be used to identify the interactions holding the structure together?

Do you think this type of interaction is given a particular name/label? (If so, how would you label this type of interaction?)
Describe this interaction in your own words. Give as much detail as you can.
Answers
Classifying interactions as attraction, force, bonding or chemical bond In question the interaction between the nucleus and the electron is an attraction, based on electrical forces, but is not usually referred to as bonding (although the term binding may sometimes be used), and is not classified as a chemical bond.
In questions 2–10: the interactions shown are examples of bonding and can be classed as chemical bonds, as well as being attractions based upon electrical forces. (Teachers will have their own expectations about the level of detail appropriate in the responses from their groups.)
- No specific name – intra-atomic forces or just electrical forces/electrostatic. Description – electrical attraction between positive nucleus and negative electron.
- Covalent bond. Description – a negative pair of electrons between the two positive nuclei binds them together. Some students may discuss the formation of a (molecular) bonding orbital from the overlap of atomic orbitals.
- a) and b) Ionic bond. Description – electrical attraction between each ion and the surrounding counter ions – ie positive cations attracted to and by surrounding negative anions – and vice versa.
- Hydrogen bonding (and van der Waals forces and dipole-dipole forces). Description – due to the difference in electronegativity between oxygen and hydrogen the bonds in water are polar (Hδ+and Oδ–). Hydrogen bonding is formed when the (δ+) hydrogen centre of one molecule is attracted to and attracts the (δ–) oxygen centre of another. A lone pair (ie non-bonding pair) of electrons on one molecule attracts and is attracted by a poorly shielded proton on another molecule.
- Solvent-solute interactions/solvation forces/hydration forces. Description – the negative poles of water molecules attract and are attracted to, the positive cations (and the positive poles of water molecules attract, and are attracted to, the negative anion).
- Covalent bond. Description – a negative pair of electrons between the two positive cores binds them together. Some students may discuss the formation of a (molecular) bonding orbital from the overlap of atomic orbitals.
- Van der Waals forces. Description – the synchronisation of transient fluctuating dipoles leads to induced dipole - induced dipole forces between molecules.
- Metallic. Description – the delocalised electrons attract, and are attracted to, the positive atomic cores. (Some students may refer to the overlap of atomic orbitals to form extensive molecular orbitals – ie the conduction band. Other students may be aware that bonding in transition metals can be considered to have covalent character in addition to its metallic nature.)
- Dative /co-ordinate bonding. Description – a lone pair of electrons on the chlorine centre from one molecule attracts, and is attracted by, the poorly shielded positively charged aluminium core of the other molecule – and vice versa.
- Covalent bond. Description – a negative pair of electrons between the two adjacent positive carbon cores binds them together. Some students may discuss the formation of (molecular) bonding orbitals from the overlap of atomic orbitals.
Notes
For the full version of this chapter, see downloads below.
Downloads
Interactions
PDF, Size 0.68 mb
Websites
Additional information
These resources have been taken from the book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 2, by Keith Taber.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
 Currently
reading
Currently
reading
Interactions
- 32
- 33
- 34
- 35
- 36





























































































No comments yet