Here’s everything you’ll ever need to teach this topic successfully
Polymers are versatile substances that can be made into a wide range of useful materials, from non-stick frying pans to bulletproof vests, with many of their potential uses yet to be discovered. They can also be found in nature in the form of proteins and DNA. Polymers can be synthesised by addition or condensation reactions and their properties can be explained in terms of intermolecular forces and functional groups.
Download this
Infographic poster, fact sheet and student worksheet. Display the poster in your classroom or on a projector. Alternatively, print it and use it as a handout.
Use the accompanying resource to test learners’ understanding of how addition polymers are structured and named. The extension question explores the properties of a common addition polymer while assessing the benefits and drawbacks of its use.
Synthesising polymers

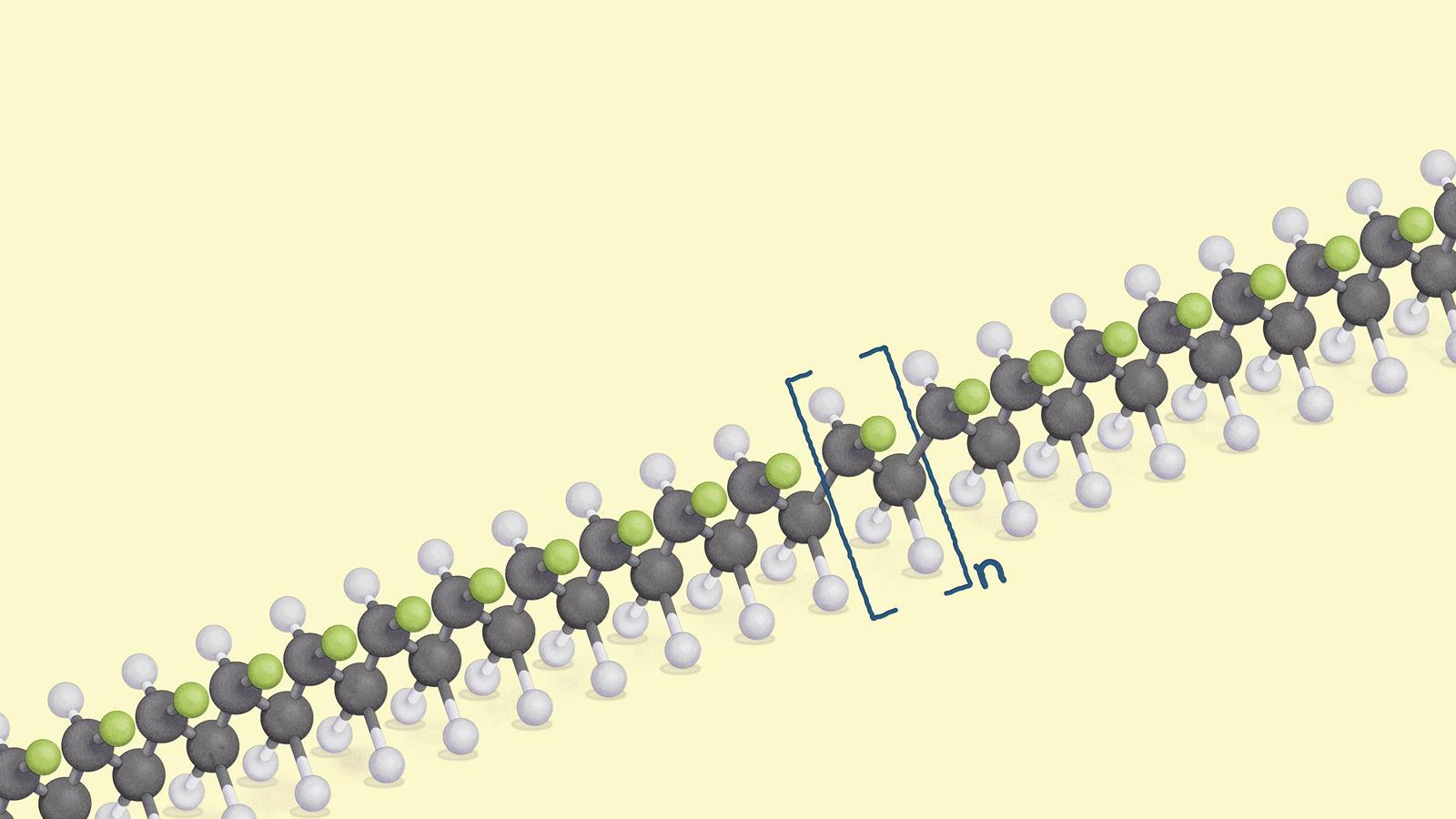
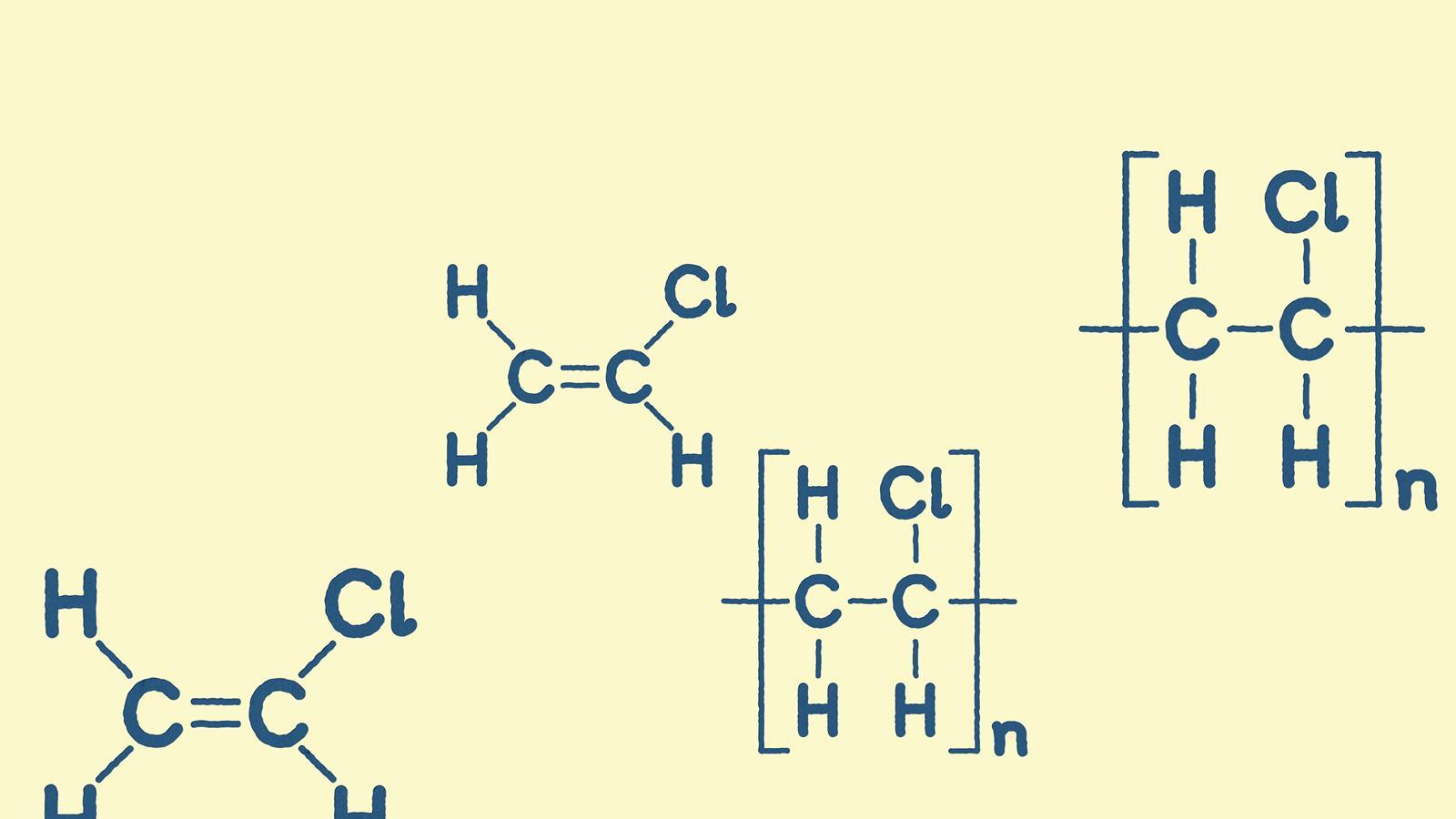
Addition polymers are macromolecules, long chain covalent molecular structures consisting of repeating units. This ball-and-stick model of chloroethene is an example of a repeating unit
In a copolymer there is more than one repeating unit. In homopolymers, there is only one, as in this ball-and-stick model of a polymer chain of poly(chloroethene) – or PVC
Structure of chloroethene as a molecule and as a repeating unit for poly(choloroethene) or PVC
Addition polymers are synthesised from unsaturated monomers without the formation of any side products. They tend to degrade less easily than condensation polymers because of the stronger C–C bonds in their backbone chain.
Physical properties
The physical properties of polymers are dictated by intermolecular forces. Hydrocarbon polymer chains are held together by London dispersion forces. Stronger polymer substances are held together by hydrogen bonding or covalent cross-links.
Did you know…? Polymers are used as thickening agents in food sauces, paints, cosmetics and inks – anything you want thickened, so it’s no surprise they are also lubricants!
Did you know…? Polylactic acid is one of the most widely used bioplastics. It is made from plant starch and is used to make disposable cutlery and the plastic for 3D printing
Did you know…? Shape memory polymers are smart materials which can use their shape memory to enable clothing to actively regulate your temperature or wound dressings to release drugs with changes in pH
More resources
- Extend your learners’ knowledge of addition polymers with this collection of differentiated worksheets.
- Use this series of activities in a sequence of lessons to mould your learners’ understanding of monomers and polymers.
- Watch Robert’s video job profile to find out how chemists develop polymers for consumer products such as cosmetics and adhesives.
Synthetic and natural polymers
Synthetic polymers, known as plastics, can take decades to degrade and can release toxic fumes if burnt. Biodegradable plastic is broken down by microorganisms into compounds found in nature, while compostable plastic will only degrade under specific physical conditions.
Naturally occurring polymers include cotton, starch, DNA and proteins. Early synthetic polymers were chemically modified natural polymers, made by nitrating cellulose (nitrocellulose) and cross-linking natural rubber (vulcanised rubber).
All illustrations © Dan Bright. Resources by Duncan Short
Want more inspiration?
Ideas for teaching polymers and polymerisation:
- Everything you need to teach polymers at 14–16
- How to use models to boost understanding
- Teach complex concepts easily with paper prompts
Posters and activities for 14–16 learners on:
Downloads
Addition polymers poster
Handout | PDF, Size 4.54 mbAddition polymers fact sheet
Handout | PDF, Size 0.12 mbAddition polymers student worksheet
Handout | PDF, Size 0.2 mbAddition polymers teacher notes and answers
Handout | PDF, Size 0.18 mbAddition polymers fact sheet
Editable handout | Word, Size 0.44 mbAddition polymers student worksheet
Editable handout | Word, Size 0.52 mbAddition polymers teacher notes and answers
Editable handout | Word, Size 0.48 mb














4 readers' comments