Watch this
Watch the video and download the technician notes from the Education in Chemistry website: rsc.li/3oBNyqC
When teaching the reactivity series, it’s common for pupils to undertake a practical to explore the patterns in the reactions of acids with the less reactive metals. Similarly, they might see demonstrations of the more reactive metals with water or even explore these themselves practically. This approach gives some pupils the impression that the two types of reactions are disparate. As a mid-reactivity series metal, magnesium can be used to help students draw a mental bridge between these reaction types. Magnesium ribbon reacts in a satisfying manner with strong acids and barely reacts at all with room temperature water. It will, however, react rapidly with steam.
Kit
- Safety screens
- Magnesium ribbon (flammable) – approximately 10 cm
- Mineral wool
- Borosilicate boiling tube
- Clamp and stand
- Rubber bung fitted with glass tubing
- Eye protection for audience
- Splash-proof goggles for demonstrator
- Bunsen burner
- Splint
Preparation
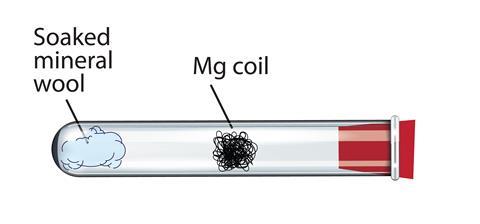
Set up safety screens to protect the audience and demonstrator. Wrap the magnesium ribbon into a coil approximately 0.5 cm in diameter and 3 cm in length. Load the mineral wool into the boiling tube and soak it with water before clamping the tube horizontally and inserting the magnesium coil. Finally, add the bung fitted with glass tubing. The end of the tubing should protrude at least 2 cm from the rubber to enable the evolved hydrogen from the demonstration to be lit.
In front of the class
The audience should remain at least 2 metres away, wearing eye protection. The demonstrator should wear splash-proof goggles. Heat the tube with a Bunsen burner directly below the magnesium until the ribbon just catches fire. Then, move the Bunsen burner to the water-soaked mineral wool to begin vaporising the water. The magnesium glows more brightly as the steam passes over it and a splint can be used to light the evolved hydrogen at the end of the glass tubing.
Safety
- Wear splash-proof goggles and use safety screens to protect both audience and demonstrator. Students should remain 2 m away and wear eye protection.
- Never look directly at burning magnesium.
- Do not use magnesium powder.
- Never attempt to react calcium or the alkali metals with steam.
- Take steps to prevent theft; never leave reels of magnesium in the laboratory.
- CLEAPSS members should consult HC016.
Teaching goal
Students will likely already have seen the reaction of magnesium with acids to produce hydrogen gas and a salt (Equation 1). They may also have seen the reaction of lithium with water to produce hydrogen gas and a hydroxide, as evidenced by the use of a universal indicator or phenolphthalein (Equation 2).
Equation 1: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Equation 2: 2Li(s) + 2H2O(l) → 2LiOH(aq) + H2(g)
This demonstration shows how the reaction of metals with water can be dramatically sped up by an increase in temperature. Students can see the production of the hydrogen gas and draw a mental connection between the reactions seen in Equation 1 and Equation 2.
Here, the reaction initially produces magnesium oxide (Equation 3), which can continue to produce the hydroxide on reaction with liquid water (Equation 4).
Equation 3: Mg(s) + H2O(g) → MgO(aq) + H2(g)
Equation 4: MgO(s) + H2O(l) → Mg(OH)2(aq)
Students could then be invited to make predictions about what the reactants and products are for the reaction of magnesium and water at room temperature and what evidence we might collect for the reaction taking place (Equation 5) if they had several days to wait for the results.
Equation 5: Mg(s) + 2H2O(g) → Mg(OH)2 (aq) + H2(g)
Having seen the use of an indicator in metal–water reactions and the production of hydrogen gas in metal–acid reactions, students might suggest these as possible signs that the reactions are connected.
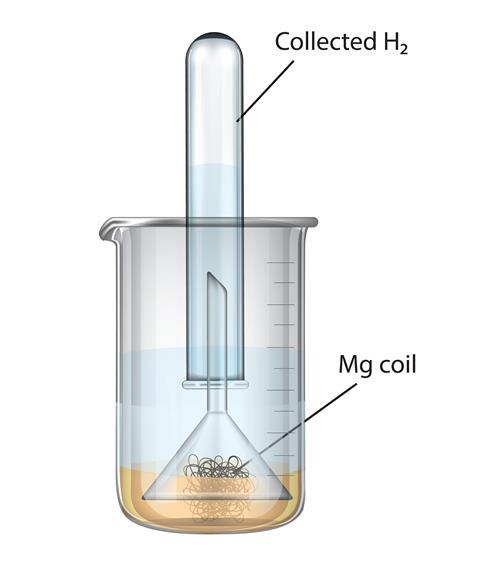
You can test this by leaving an inverted funnel and collecting tube over some magnesium ribbon which has been submerged in water with a few drops of phenolphthalein. The indicator will begin to change colour within a few minutes (Figure 2) but a few days may be needed to collect a significant volume of gas which could be tested in the next lesson.
Disposal
Where the burning magnesium was in contact with the glass, magnesium silicide may have been produced. The boiling tube should not be reused but can be rinsed in 500 cm3 of water to convert any silicides to silanes. Small pops or flashes from pyrophoric silanes may be seen. The rinsed glassware can be disposed of in the broken glass bin.
Downloads
Technician notes
Editable handout | Word, Size 0.18 mbTechnician notes
Editable handout | PDF, Size 0.18 mb

























No comments yet