It seemed to me when I was at school, that ‘larger surface area’ was the universal answer to all GCSE science questions. If I was feeling particularly brave or lazy then I figured that writing this in response to any question might, on a good day, earn me a C! With age and wisdom, I’ve come to realise how important the surface area of a material can be in terms of how it behaves. Take sugar, for example; it’s a seemingly harmless, edible material, but under certain circumstances, it can level a factory. Like the chip pan fire demonstration, the dust explosion demo is useful for demonstrating rates of reaction but, also, showing it to people could save a life.
It seemed to me when I was at school, that ‘larger surface area’ was the universal answer to all GCSE science; If I was feeling particularly brave or lazy then I figured that writing this in response to any question might, on a good day, earn me a C! With age and wisdom, I’ve come to realise how important the surface area of a material can be in terms of how it behaves. Take sugar, for example; it’s a seemingly harmless, edible material. But under the right circumstances, it can level a factory. Like the chip pan fire demonstration (rsc.li/3SfyEUH), the dust explosion demonstration is useful for demonstrating rates of reaction but, also, showing it to people could save a life.
Kit
- 500–750 g catering tin of coffee (or similar) with a soft resealable lid, usually low-density polyethylene (LLDPE) marked recycling code 4
- A few grams of icing sugar / custard or lycopodium powder / cornflour
- Sieve
- Tea light
- Tubing
- Bulb pipette filler or modelling balloon pump
Preparation
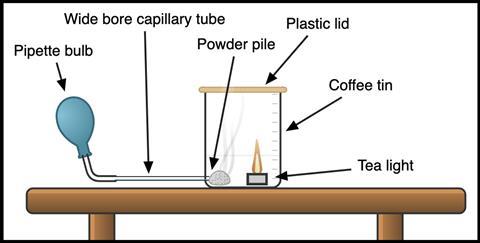
The objective is to end up with a tin that has a hole in the side, with a tube fed through to enable the powder to be puffed into the air above a lit tea light (see Figure 1).
CLEAPSS members should consult SRA002 for different ways of accomplishing this. My go-to technique involves boring a hole at the base of the tin with a pair of scissors, just wide enough to pass through a 5–6 mm outside diameter tube. The tube should have an internal diameter of approximately 3 mm to minimise powder blowback. By threading the tubing through a rubber stopper, you can help maintain a good seal with the tin and this allows you to test different arrangements of powder and candle. I find that puffing the powder in the direction of the candle (as shown in Figure 1) provides a more reliable, but smaller pop. CLEAPSS recommends using a small funnel loaded with powder, or running the tube to the opposite side of the tin so the powder is puffed up and back towards the flame. This makes for a larger flame when it works, at the cost of reliability.
The objective is to end up with a tin that has a hole in the side, with a tube fed through to enable the powder to be puffed into the air above a lit tea light.
CLEAPSS members should consult SRA002 (bit.ly/3MNbjc3)for different ways of accomplishing this. My go-to technique involves boring a hole at the base of the tin with a pair of scissors, just wide enough to pass through a 5–6 mm outside diameter (OD) tube. The tube should have an internal diameter (ID) of approximately 3 mm to minimise powder blowback. By threading the tubing through a rubber stopper, you can help maintain a good seal with the tin and this allows you to test different arrangements of powder and candle. I find that puffing the powder in the direction of the candle provides a more reliable, but smaller pop. CLEAPSS recommends using a small funnel loaded with powder, or running the tube to the opposite side of the tin so the powder is puffed up and back towards the flame. This makes for a larger flame when it works, at the cost of reliability.
Although other powders also work, I use icing sugar because it’s widely available and the combustion by-products produce a pleasant smell. The powder needs to be bone dry – as these powders often lie open for extended periods in a prep room, it is worthwhile drying in an 80°C oven and sieving afterwards to remove any caked powder.
Safety and disposal notes
- Always practise in advance. Even an experienced teacher can be surprised by this reaction working better or worse than expected. The nature of this kit is such that you may need to remake it because the lid can become looser over time, so the initial containment is less effective.
- This demonstration is suitable for adults but should not be conducted by pupils.
- CLEAPSS members should consult SRA002. Do not exceed the quantities outlined above or deviate from the method.
- There is an inhalation risk associated with the powders used in this demonstration – especially if using lycopodium (which consists of spores like pollen that may be sensitising). After a reaction, or a failed reaction, take care to not raise unreacted dust when disposing of the remains.
In front of the class
Set up safety screens to protect the demonstrator and audience. Place the screens close to the can so the lid can only escape vertically. Wear eye protection and ensure students are at least two to three metres away.
Dim the lights to enhance the effect and help students keep track of the candle, as it is out of view inside the tin. I use a spoon to gather the powder around the end of the tube to ensure it’s covered. Light the candle and firmly close the lid.
Immediately squeeze the pipette bulb or syringe to puff up the powder into the air above the candle. The powder rapidly combusts, causing a flash and the lid to fly off with a pop.
It is possible to get repeated reactions, but over time the candle can become clogged with powder so it’s handy to have a spare to hand.
Teaching goal
As well as acting as an alternative to the howling jelly baby as a demonstration of the energy content of food, this is also a popular demonstration to illustrate the importance of surface area to rates of reaction (see also pyrophoric carbon). Lycopodium powder has a relatively high fat content and can be used to generate a bright flame for use in theatrical and photographic flash powders. The most infamous example of this reaction has to be the dust explosion.
Reports of dust explosions in flour warehouses date back to 1785 and, sadly, these explosions continue to take lives. Other combustible powders such as plastics and aluminium are also culprits. In an explosion at a Georgia sugar refinery in 2008, 14 people were killed and this sparked an international focus on this kind of accident in the US. The US Chemical Safety Board conducted a major investigation and found that dust explosions had played a role in 281 incidents in the US over the 25-year period prior to 2008.
As well as acting as an alternative to the howling jelly baby (rsc.li/3gnm7Bs) as a demonstration of the energy content of food, this is also a popular demonstration to illustrate the importance of surface area to rates of reaction. Lycopodium powder has a relatively high fat content and can be used to generate a bright flame for use in theatrical and photographic flash powders. The most infamous example of this reaction has to be the dust explosion.
Reports of dust explosions in flour warehouses date back to 1785 and, sadly, continue to take lives, along with other combustible powders such as plastics and aluminium. In an explosion at a Georgia sugar refinery in 2008, 14 people were killed and this sparked an international focus on this kind of accident in the US. The US Chemical Safety Board conducted a major investigation (bit.ly/3sddFHq) and found that dust explosions had played a role in 281 incidents in the US over the 25 year period prior to 2008.
It’s worth discussing the key requirements for such an explosion to occur if your first attempt does not work in front of the class. The coffee tin demonstrates how the right combination of oxygen and dust must be present, and the lid also simulates another key requirement: some kind of containment. While students will be familiar with product versus time curves showing reactions decreasing in speed, an explosion needs to do the opposite. An element of containment can allow for temperature and pressure to rise, compensating for any reduction in local reactant concentration. In the worst of accidents, the shockwave from one explosion raises dust in neighbouring parts of a factory, leading to a sequence of explosions.
Declan Fleming

























No comments yet