Try this demonstration to produce some spectacular exothermic redox reactions by reacting aluminium with halogens
In this experiment, students observe the reactions that occur when aluminium reacts with each of three halogens – chlorine, bromine and iodine. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides:
2Al + 3X2 → 2AlX3 (X = Cl, Br and I).
The demonstration provides a vivid illustration of the reactivity of three non-metals from Group 17 with a metal.
These experiments must be done in a fume cupboard as both the reactants and the products are hazardous. Teachers attempting this demonstration for the first time are strongly advised to do a trial run before doing it in front of a class.
Each experiment should take about five minutes.
Equipment
Apparatus
- Eye protection (goggles)
- Thick chemically-resistant gloves such as marigold industrial blue nitrile
- Access to a fume cupboard
- Mortar and pestle
- Heat resistant mat, 30 x 30 cm
- Watch glasses, about 10 cm diameter, x2
- Reduction tube (see note 10 below)
- Test tubes, x3
- Test tube rack
- Teat pipette
- Filter paper
- Spatula or wooden splint
- Bosses, clamps and stands
Chemicals
- Aluminium foil, a few cm2
- Aluminium powder (HIGHLY FLAMMABLE, CONTACT WITH WATER MAY LIBERATE HYDROGEN), 0.1 g
- Liquid bromine (CORROSIVE, VERY TOXIC), 1 cm3 (see note 4 below)
- Solid iodine (HARMFUL), 0.4 g
- Silver nitrate solution, about 0.1M, about 10 cm3
- A little deionised water in a wash bottle
- Chlorine generator (TOXIC, IRRITANT) (see note 7 below)
- Sodium chlorate(I) solution (sodium hypochlorite) (14% (w/v) available chlorine) (CORROSIVE), about 100cm3
- Hydrochloric acid, 5 M (CORROSIVE), about 50 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection (goggles) and heavy duty gloves (due to the corrosive nature of liquid bromine).
- Aluminium powder – see CLEAPSS Hazcard HC001A.
- Liquid bromine – see CLEAPSS Hazcard HC015a. Wear suitable protective gloves (thick, chemically resistant) when handling liquid bromine. Have 500 cm3 of 1M solution of sodium thiosulfate available to treat any spillages on the skin.
- Solid iodine – see CLEAPSS Hazcard HC054.
- Silver nitrate solution – see CLEAPSS Hazcard HC087.
- Chlorine – see CLEAPSS Hazcard HC022a and Recipe Card RB024, plus guidance on chlorine from these standard techniques for generating, collecting and testing gases.
- Sodium chlorate(I) solution – see CLEAPSS Hazcard HC089.
- Hydrochloric acid – see CLEAPSS Hazcard HC047a.
- The reduction tube should be fitted with a one-holed rubber stopper fitted with short length of glass tubing and attached to the chlorine generator. Alternatively an 8–10 cm length of wide bore glass tubing with a stopper fitted with a short length of glass tubing at each end could be used – see the diagram below.
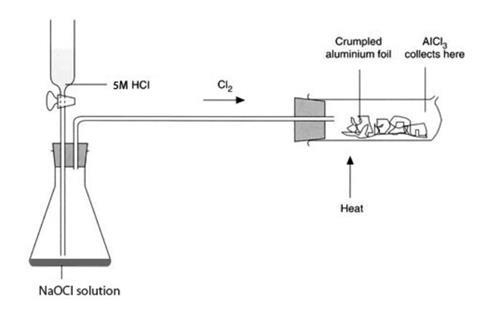
Procedure
Reaction of Al + Cl2
- Set up the chlorine generator in a fume cupboard. Make sure it is securely clamped.
- Loosely crumple a piece of aluminium foil (10 x 5 cm) so that it will just fit inside the reduction tube and push it into the tube. Attach the tube to the generator with a short length of rubber tubing and clamp it in position at the end nearest to the generator, so that the aluminium foil can easily be heated using a Bunsen burner – see diagram.
- Generate a gentle stream of chlorine by dripping the hydrochloric acid slowly on to the sodium chlorate(I) solution, and allow it to pass over the aluminium. When the green colour of the chlorine gas fills the reduction tube, start heating the aluminium foil with a Bunsen burner at the end nearest to the chlorine supply. Once the aluminium is hot, a bright glow will suddenly appear where it starts to react with chlorine.
- Remove the heat. The bright glow should spread along the aluminium. If not, heat again, and increase the flow of chlorine gas. A lot of white ‘smoke’ – aluminium chloride – is produced, some of it condensing as a white powder on the walls of the reduction tube and the rest streaming out of the hole in the end of the tube. When the reaction is over, stop the chlorine supply and remove the heat.
- When the reduction tube has cooled down, disconnect it and, still in the fume cupboard, scrape a little of the white powder into a test tube. Add a little deionised/distilled water to the solid to dissolve it. Care: the reaction between anhydrous aluminium chloride and water can be quite vigorous – an audible hiss can often be heard (see Teaching notes below).
- Test a drop of the solution with Universal indicator paper. It is strongly acidic. Test the remainder with a little silver nitrate solution. A white precipitate forms, showing the presence of chloride ions.
Reaction of Al + Br2
- Tear or cut some aluminium foil into several small pieces about 2 x 2 mm in size. Carefully pour 1 cm3 of liquid bromine onto a watchglass on a heat resistant mat in a fume cupboard. Sprinkle a few pieces of aluminium foil on to the surface of the bromine. Within a minute there are flashes of flame and a white ‘smoke’ of aluminium bromide is formed, together the orange vapour of bromine. Carefully hold another watchglass over the reaction to condense some of the ‘smoke’ on to its surface as a solid.
- Wash any aluminium bromide collected in this way off the watchglass into a test tube using a little deionised water (care: see Teaching notes below). Test the solution with indicator paper and silver nitrate solution as above. The solution is acidic and a cream precipitate of silver bromide is formed.
Reaction of Al + I2
Note
The quantities specified in the reaction of Al + I2 should not be exceeded unless the school has an Explosives Certificate issued by the police.
- Weigh out 0.4 g of iodine, which should be dry, and grind it to a powder in a fume cupboard, using a mortar and pestle. Place the powdered iodine on a filter paper on a dry heat resistant mat and add 0.1 g of aluminium powder to it. Mix the two solids together in the fume cupboard using a wooden splint – do NOT grind them together. When they are thoroughly mixed, pour the mixture into a heap on the heat resistant mat or in a metal tray, such as a tin lid, positioned in the middle of the fume cupboard.
- To start the reaction, use a teat pipette to place a few drops of water on the mixture. After a time lag, the water begins to steam and clouds of purple iodine vapour are given off, indicating that an exothermic reaction has started. After a few more seconds sparks are given off and the mixture bursts into flame. When the reaction subsides, a white residue of aluminium iodide remains. Scrape a little of this into a test tube (care: see Teaching notes below), add some deionised water and filter if necessary. Test the solution with indicator paper and silver nitrate solution as above. The solution is acidic and a yellow precipitate indicates the presence of iodide ions.
Teaching notes
These reactions make quite spectacular demonstrations in themselves, the bromine and aluminium reaction even more so in a partly darkened room. Classroom management in semi-darkness (You need to plan carefully for such lessons. Ensure that students are clear about what they need to do during such activities.)
The demonstrations can be used to show the reaction between reactive non-metallic elements and a fairly reactive metal to form compounds, or as part of the study of the reactions of the Group 17 elements. Here the apparent order of reactivity is not that predicted from their position in the Group (that is chlorine → bromine → iodine). This is because of the different physical states of the three halogens, and the different surface area of the aluminium as a powder or foil. This can be used to make an important point about ‘fair’ comparisons of reactivity.
These reactions also serve to show that aluminium is in fact a more reactive metal than it appears in everyday use. The protective oxide layer of aluminium has to be penetrated by the halogens before the reactions can start, hence the delays, and the need for water to assist the two solid elements getting into contact, in the case of aluminium and iodine.
The clouds of iodine vapour released when aluminium and iodine react can stain the inside of a fume cupboard. Teachers may prefer to demonstrate this reaction outdoors, if possible.
The anhydrous aluminium halides are vigorously hydrolysed (sometimes violently if freshly prepared and hot, as here) by water, giving off fumes of a hydrogen halide and a forming an acidic solution of the aluminium salt. To dispose of the solid residues, allow them to cool completely before adding in small amounts to 1 M sodium carbonate solution in a fume cupboard. Wait until the reaction has subsided before adding more solid. Dispose of the resultant slurry with plenty of water.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet