Gases give rise to particular hazards so great care must be taken when preparing, collecting or testing
Gases give rise to particular hazards so great care must be taken when preparing, collecting or testing them.
How the gas is to be used will differ from experiment to experiment – it is essential to read carefully the specific instructions given or referred to in the experimental procedure and any accompanying technical notes. This is especially important if the gas needs to be dried.
Gases can be collected by upward or downward delivery or over water. Refer to specific information on each gas below.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye and skin protect if required.
- Hydrochloric acid is highly corrosive, refer to CLEAPSS Hazcards HC047a, plus CLEAPSS Recipe Book RB021.
- Zinc is of low hazard, refer to CLEAPSS Hazcard HC108b
- Copper is of low hazard, refer to CLEAPSS Hazcard HC026.
- Hydrogen gas is EXTREMELY FLAMMABLE – ensure there are no naked flames. Refer to CLEAPSS Hazcard HC048
- Hydrogen peroxide (IRRITANT) to manganese(IV) oxide powder (HARMFUL). Collect the gas over water. Refer to CLEAPSS Hazcard HC050
- Oxygen is an OXIDISING AGENT, refer to CLEAPSS Hazcard HC069
- Potassium manganate(VII) (OXIDISING, HARMFUL and DANGEROUS FOR THE ENVIRONMENT). Refer to CLEAPSS Hazcard HC081
- Double-check that the acid is hydrochloric and NOT sulfuric.
- Sodium chlorate(I) can cause headache, fatigue, dizziness, and methemoglobinemia. Refer to CLEAPSS Hazcards HC089.
- Chlorine is classified as toxic, irritant and dangerous for the environment. Refer to CLEAPSS Hazcard HC022a
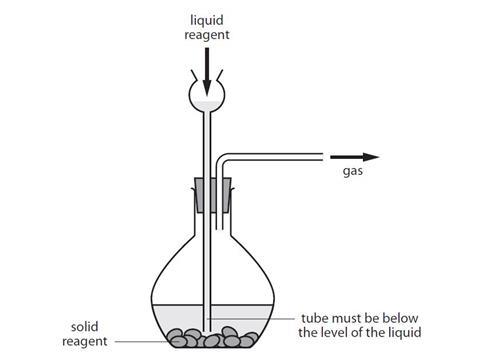
General gas preparation
The diagram below shows a typical set of apparatus which can be used to prepare a range of gases.
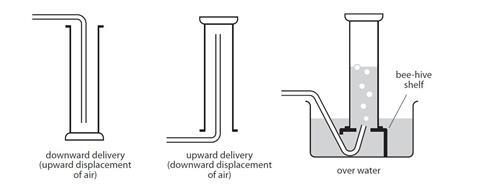
Gas collection methods
The diagrams below show three different methods for collecting gas.
Preparing specific gases
Wear appropriate eye protection. The amounts given below are sufficient to generate 1 litre (1 dm3) of each of the named gases.
Carbon dioxide, CO2
Slowly add 42 cm3 of 2 M hydrochloric acid (IRRITANT) to an excess of marble chips. Collect the gas by downward delivery or over water (slightly soluble).
Hydrogen, H2
Slowly add 28 cm3 of 3 M hydrochloric acid (CORROSIVE) to excess zinc granules and 1 g of hydrated copper sulfate (HARMFUL). Collect the gas by upward delivery or over water.
Hydrogen gas is EXTREMELY FLAMMABLE – ensure there are no naked flames.
Oxygen, O2
Slowly add 50 cm3 of 20 vol hydrogen peroxide (IRRITANT) to manganese(IV) oxide powder (HARMFUL). Collect the gas over water.
Oxygen is an OXIDISING AGENT.
Chlorine, Cl2
Work in a fume cupboard. Method 2 is safer and recommended but slower.
Method 1
Add 14 cm3 of concentrated hydrochloric acid (CORROSIVE) to at least 3 g of potassium manganate(VII) (OXIDISING, HARMFUL and DANGEROUS FOR THE ENVIRONMENT).
Double-check that the acid is hydrochloric and NOT sulfuric.
Method 2
Add 5 M hydrochloric acid (IRRITANT) to 30 cm3 of recently purchased (10–14% available chlorine) sodium chlorate(I) solution (CORROSIVE) with plenty of stirring. Note that sodium chlorate(I) is only available as a solution often called ‘sodium hypochlorite’; it must not be confused with sodium chlorate(V) (sometimes just called ‘sodium chlorate’), which is a white, crystalline solid. School samples often react too slowly because old sodium chlorate(I) is used. This will have less than the required 10% available chlorine (as it applies to both methods).
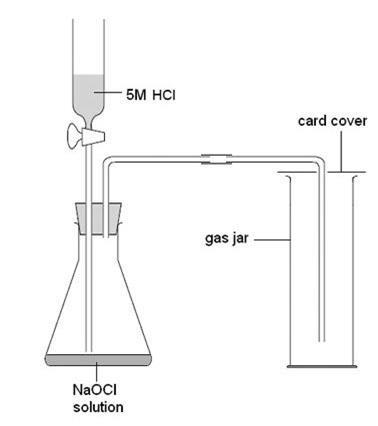
Collect the gas by downward delivery. Chlorine is classified as TOXIC, IRRITANT and DANGEROUS FOR THE ENVIRONMENT.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The resource is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















3 readers' comments