Share this infographic with your students, download the poster for your classroom and get students developing their knowledge of changes of state and mixtures
-

Infographic poster and fact sheet
Display the poster in your classroom or on a projector. Alternatively print it and use as a handout.
- Poster as pdf A4 single pages or A3 one page
- Fact sheet as MS Word or pdf
-
Melting chocolate activity
Use the accompanying fact sheet and activity to explore cooling ranges of mixtures and develop graphing skills. It also offers an alternative context to the stearic acid practical and is a great opportunity to apply that learning to a new set of data.
View and download more infographics
Did you know chocolate begins to melt at a temperature lower than the temperature of the human body? That’s why when you put some in your mouth it begins to melt.
So what is the melting point of chocolate? There’s isn’t an exact point. There’s a range, because it’s a mixture.
What is chocolate made from?
Cocoa is the simple answer. Cocoa comes from the seed pods of cocoa trees. The seed pods contain beans, which are fermented, roasted and processed. Other ingredients, such as sugar and milk, are added to make the finished chocolate.


Cocoa comes from the beans inside the seed pods of cocoa trees. These beans contain roughly 50% cocoa butter, which is chocolate’s main ingredient. Cocoa butter is made up of three fats in roughly equal amounts. The ratio of these fats strongly affects chocolate’s melting range.
To make chocolate melt in your mouth, chocolatiers try to maximise the amount of Type V fat crystals in the mixture using a process called tempering. This involves heating and cooling the mixture to melt the different crystal types (which have different melting points) until mainly Type V remains.
Crystallisation
The fats in cocoa butter can form six different types of crystal, which melt at different temperatures:
| Crystallisation type | Melting point | Taste notes |
|---|---|---|
|
Type I |
17.3°C |
Soft, crumbly |
|
Type II |
23.3°C |
Crumbly, melts easily |
|
Type III |
25.5°C |
Firm but melts easily |
|
Type IV |
27.3°C |
Firmer but melts easily |
|
Type V |
33.8°C |
Best for eating: melts near body temperature, crisp snap |
|
Type VI |
36.3°C |
Too hard |
The melting range of chocolate depends on the types of crystals that chocolatiers create in the mixture.
Did you know …?
Chocolate with Type VI crystals is sometimes used to make heat-resistant chocolate for army survival packs.
Tempering
To make chocolate melt in your mouth, chocolatiers try to maximise the amount of Type V crystals in their creations using a process called tempering. This involves:
- heating the chocolate to about 40°C to make sure all the various crystal forms are melted;
- cooling it gradually to 28°C to give a mixture of Type IV and Type V crystals;
- heating it again, this time to 32°C to melt the Type IV crystals;
- then pouring it into moulds where it sets.
Melting ranges
The mixture of ingredients in chocolate recipes affects the melting point of the finished product. For example, adding milk to dark chocolate to make milk chocolate lowers the melting point.
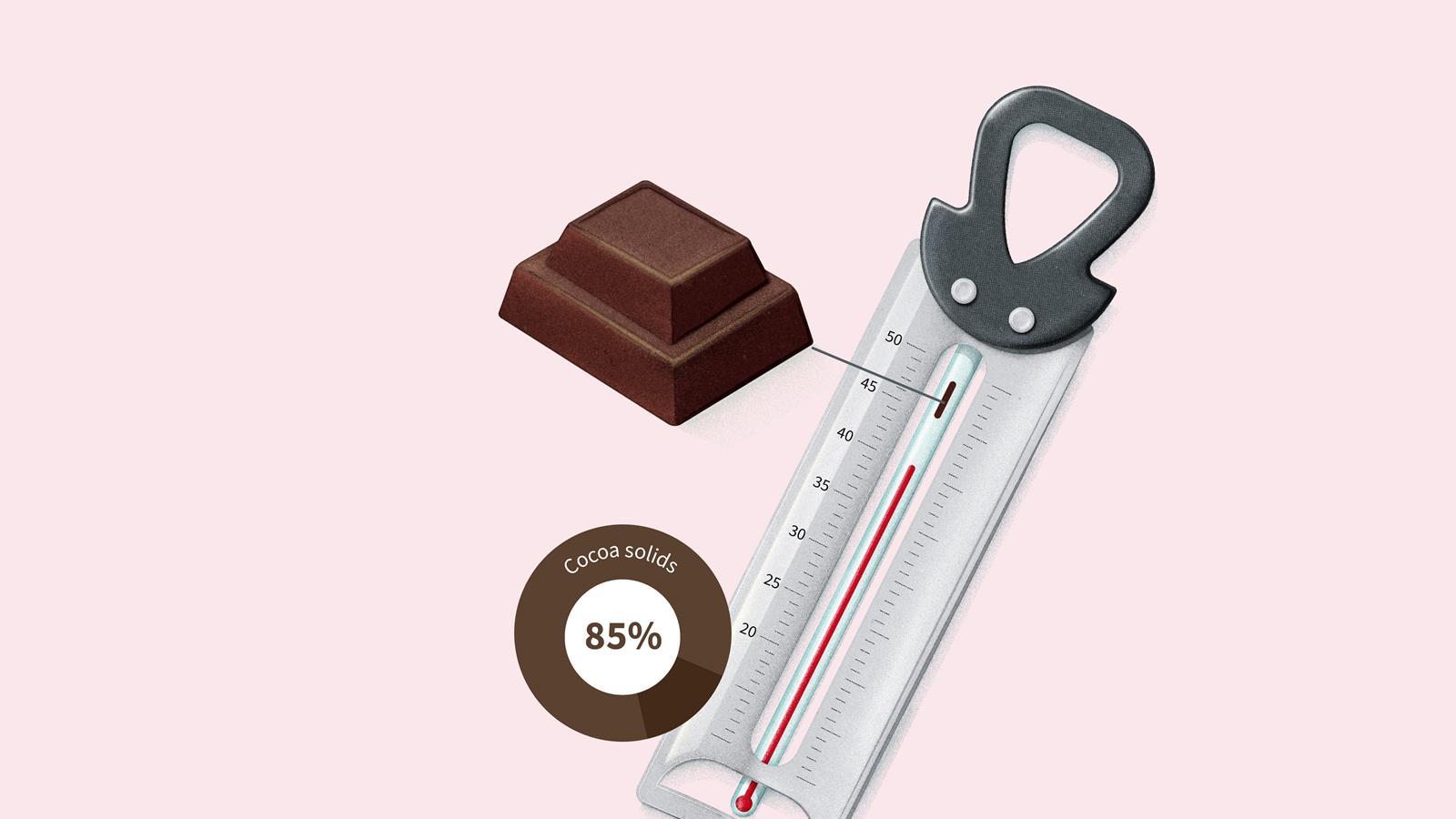

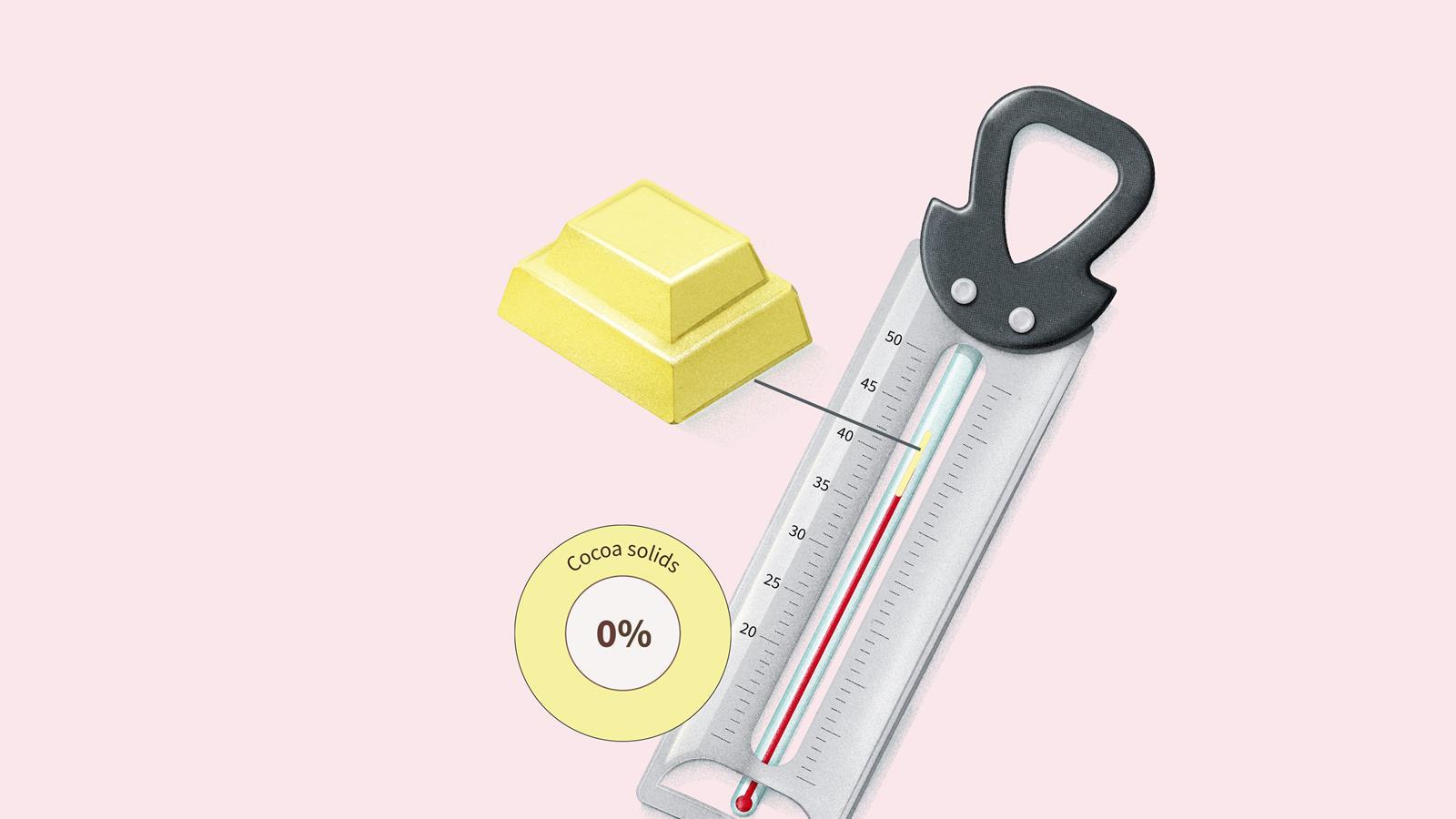
Dark chocolate with 85% or more cocoa solids doesn’t begin to melt until around 46°C.
Milk chocolate with between 20 and 50% cocoa solids melts between 40 and 45°C.
White chocolate with no cocoa solids and roughly 20% cocoa butter melts between 37 and 43°C.
Did you know …?
Dark chocolate contains theobromine, which is toxic for dogs. So don’t give them any!
All illustrations © Dan Bright. Resources by Holly Walsh
Teaching 11–14 learners?
Try these posters and activities on curriculum topics for younger students:
- Earth’s resources, with scaffolded calculations worksheet
- Earth’s structure, with DART activity
- Periodic table, plus elements activity
- Rock cycle poster, plus Rock and roll game
- Water cycle poster, with structure strips
- Evaporation poster and practical activity
- Link curriculum learning to careers by exploring Robert’s role as an associate principle scientist to influence the taste and texture of chocolate.
Downloads
Melting chocolate infographic poster A4
Handout | PDF, Size 0.38 mbMelting chocolate infographic poster A3
Handout | PDF, Size 0.38 mbMelting chocolate - worksheet and teacher notes
Editable handout | Word, Size 0.19 mbMelting chocolate - worksheet and teacher notes
Handout | PDF, Size 0.27 mbMelting Chocolate - answer sheet and mark scheme
Editable handout | Word, Size 0.39 mbMelting Chocolate - answer sheet and mark scheme
Handout | PDF, Size 0.43 mbMelting chocolate - fact sheet
Editable handout | Word, Size 75.86 kbMelting chocolate - fact sheet
Handout | PDF, Size 0.15 mb


































No comments yet