Use this practical to explore the changes in the colour and consistency of sulfur as you heat it, melt it and eventually boil it
In this experiment, students observe what happens when sulfur is heated slowly and steadily from room temperature until it melts and boils.
A fresh sample of sulfur is heated to just above the melting point, then allowed to cool and crystallise slowly as monoclinic sulfur. A further sample is heated to boiling point, and the liquid rapidly chilled in cold water to form plastic sulfur. A third sample is dissolved in a warm solvent, and the solution allowed to cool and evaporate, leaving crystals of rhombic sulfur.
All the observed changes in properties can be related to the different structural forms of the three solid sulfur samples (allotropes). Students can see how these changes in structure occur as the temperature of the liquid sulphur is gradually raised.
The practical is described here as a demonstration. However, some teachers may wish to consider whether certain parts could be used as class practicals with appropriately skilful and reliable classes.
A demonstration, without any accompanying discussion about the possible reasons for the changes in properties in terms of structure, would take up to 45 minutes. However, to derive maximum benefit from the experiment, more time needs to be allowed for such discussion.
Equipment
Apparatus
- Eye protection
- Heat resistant gloves
- Access to a fume cupboard
- Flexicam or similar camera, digital microscope, digital projector and screen (or other method of projecting images of small crystals to the class, as available)
- Boiling tubes, x4 (see note 7 below)
- Test tube holders, x2
- Test tube rack
- Stands and clamps, x2
- Conical flask, 250 cm3
- Cork, to fit conical flask
- Beaker, 250 cm3, x2
- Beaker, 1 dm3 (see note 8)
- Thermometer, 0–250 °C
- Petri dishes or watchglasses, x4 (or more)
- Bunsen burner, tripod and gauze, or electric hotplates, x2 (optional, if available)
- Heat resistant mats, x2
- Filter paper, about 18–20 cm diameter
- Spatula
- Paper clips
- Damp cloth (to extinguish small sulfur fires)
Chemicals
- Sulfur, powdered roll, 100 g
- Dimethylbenzene (xylene), (HARMFUL), 100 cm3
- Cooking oil, 700 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Sulfur, S8(s) – see CLEAPSS Hazcard HC096A. The sulfur used must be roll sulfur, crushed to a powder. To crush the rolls of sulfur, place in a strong plastic bag on a hard surface. Use a hammer or a vice to break up the roll sulfur into small pieces, then crush to a powder in a mortar and pestle. ‘Flowers of sulfur’ is not suitable because it contains a lot of insoluble amorphous sulfur.
- Dimethylbenzene (xylene), (CH3)2C6H4(l), (HARMFUL) – see CLEAPSS Hazcard HC046a. Although other hydrocarbon solvents, such as methylbenzene, can be used to dissolve sulfur and form monoclinic sulfur, dimethylbenzene (xylene) is the least hazardous.
- Cooking oil – If suitable cooking oil is not available, other clear, high-boiling oils may be used, eg paraffin oil – see CLEAPSS Hazcard HC045b.
- During the experiments sulfur may catch fire, releasing sulfur dioxide (TOXIC – see CLEAPSS Hazcard HC097), which may cause breathing difficulties to some students. If this happens, extinguish quickly by placing a damp cloth over the mouth of the test tube. If the combustion cannot be extinguished quickly, the test tube should be placed in fume cupboard, and the fan left running.
- These are large (150 x 25 mm) test tubes, and should be clean and dry. The test tubes in which sulfur has been heated can be difficult to clean for general use. It may be worth keeping a set of such tubes from year-to-year for this experiment.
- The large beaker containing the cooking oil functions as an oil-bath for heating the sulfur slowly and uniformly, while allowing students to see clearly what is happening to the sulfur. Other containers may be preferred for the oil-bath, provided the visibility is maintained, for example by use of a webcam and digital projector.
Procedure
Before the demonstration
- Preheat the oil-bath to about 130 °C, and maintain this temperature.
- Clamp one of the sulfur-containing tubes in the oil bath, so that the sulfur is below the level of the oil in the bath.
- Half fill the 250 cm3 beaker with cold water.
- In the fume cupboard, put about 10 g of powdered roll sulfur into the conical flask and add about 100 cm3 of dimethylbenzene.
- Prepare filter paper cone held together by a paper clip and supported in a beaker, as shown below:
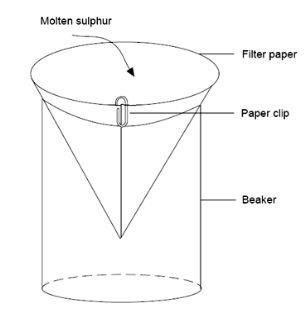
The demonstration
- Two-thirds fill two test tubes with powdered roll sulfur (about 20 g in each tube) and place in the oil bath. The sulfur will melt to a transparent, amber, mobile liquid in about 15 minutes.
- Remove one tube from the oil-bath and pour the molten sulfur into the filter paper cone. Allow the sulfur to cool slowly and solidify, forming a crust.
- Break the crust with a spatula and, handling the filter paper cone with heat resistant gloves, tilt it so that any remaining liquid flows out of the cone of solidifying sulfur on to a piece of scrap paper or card (for disposal). Needle-shaped crystals of monoclinic sulfur will be seen inside the hollow cone. When cool, the cone can be passed around the class. It may be necessary to break the cone open to see the crystals more easily.
- Over the next day or two, look carefully at the needle crystals from time to time. They will slowly go cloudy, yet retain their needle shape, as the monoclinic form slowly turns back to the more stable rhombic sulfur – each needle becomes a mass of tiny rhombic crystals.
Liquid sulfur
- Remove the second tube from the hot oil using a reliable test tube holder and wipe off any oil using a paper towel. Heat the molten sulfur gently over a small Bunsen flame, keeping the contents moving to prevent local overheating. The liquid gets darker and, fairly suddenly, becomes a viscous, gel-like substance. This occurs at about 200 °C.
- The tube can be inverted and the sulfur will remain in it. Show that the mobile liquid re-forms on cooling.
- Now heat the sulfur slowly and steadily beyond the gel-like stage. The sulfur liquefies again to a very dark red-brown liquid. Note that during this heating the sulfur may catch fire and sulfur dioxide will be produced. Have a heat resistant mat or damp cloth to hand to place over the mouth of the tube to extinguish the blue flames.
- When the sulfur begins to boil (441°C), pour the liquid sulfur in a slow stream into a beaker of cold water. A tangled mass of brown plastic sulfur will form.
- Allow this to cool thoroughly. The inside of the plastic sulfur may remain molten after the outside has solidified.
- Remove the plastic sulfur from the water and show that it is rubbery – it can be stretched and will return to its original shape.
- The shiny surface of the plastic sulfur begins to dull and some of the elasticity is lost within 30 minutes, as it begins to turn back to the more stable rhombic sulfur.
- Leave the plastic sulfur until the following lesson to monitor the progress of this change. This will be very noticeable after a week or so but complete change will take a long time. It will become brittle.
Rhombic sulfur
- Gently warm the conical flask containing sulfur and dimethylbenzene to about 50 °C (preferably on an electric hotplate) to complete dissolving of the sulfur. Some teachers may prefer to have done this before the demonstration to save time.
- Pour a little of the solution into each of a set of petri dishes or watch glasses and leave them in the fume cupboard for the solvent to evaporate. This will take about 10 minutes.
- The small crystals of rhombic sulfur formed should be viewed by projection of images onto a screen if possible.
Teaching notes
Some stages of this demonstration are time-consuming, eg melting the sulfur in the oil bath, dissolving the sulfur in dimethylbenzene, and evaporating the solvent. Some teachers may prefer to melt some sulfur before the lesson and to prepare rhombic crystals before the lesson to save time. In the latter case, slower evaporation (which can be brought about by covering the petri dish with filter paper with a few holes in) will produce larger crystals. Particularly large and/or well-formed crystals could be retained as examples for future use.
Monoclinic crystals can be formed by allowing a hot solution of sulfur in boiling dimethylbenzene to cool so that crystallisation starts at above 96 °C.
Carbon disulfide has been use in the past as a better solvent for making rhombic sulfur, however its smell, toxicity and high flammability make it unsuitable for use in schools – see CLEAPSS Hazcard HC020b.
Very slow heating is essential if all of the changes on heating sulfur are to be seen clearly. Sulfur is a poor thermal conductor, hence the changes can overlap one another if the heating is too fast. It is difficult to heat slowly enough using a Bunsen burner – hence the use of an oil bath.
Crystalline sulfur consists of puckered S8 rings in the shape of crowns. These can be packed together in two different ways – to form rhombic crystals and to form needle-shaped monoclinic crystals, as shown below:
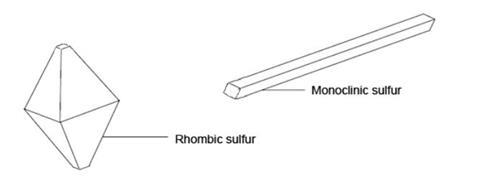
Below about 96 °C, rhombic sulfur is the more stable allotrope. On melting at about 118 °C, sulfur first forms a mobile, amber liquid containing S8 rings. If this is allowed to cool, monoclinic sulfur forms as crystallisation occurs above 96 °C.
Monoclinic sulfur will turn slowly into the more stable rhombic form on standing below 96 °C.
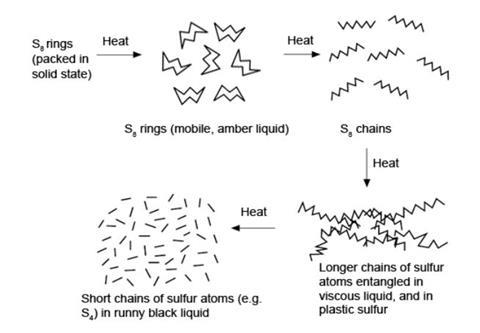
Further heating of the S8-containing liquid breaks the rings into S8 chains. These may join to form longer chains which tangle, causing an increase in viscosity. At higher temperatures, these chains break into shorter ones, perhaps as short as S2, and the viscosity decreases again.
Rapid cooling of this liquid traps the resulting solid sulfur in the tangled chain state – this is plastic sulfur. On stretching, the chains uncoil and on releasing the tension they return to the partly coiled state (see the diagram below).
If solid sulfur is formed below 96 °C by crystallisation from a solution, the stable rhombic form is produced.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry
Health and safety checked, 2016


















No comments yet