This chapter provides a brief introduction to the topic of learners’ ideas in science
Exploring how learners construct ideas around chemistry, and how to work with learners to avoid misconceptions.
The nature of learners’ ideas
The research into learners’ ideas about scientific topics suggests that students’ informal ideas vary across a number of dimensions. Some of the ideas reported appear to be quite specific, whilst others appear to be more general.
The advice given to teachers can be summarised in three points:
- In any class, for any science topic, students are likely to hold a wide range of alternative ideas about the topic.
- Not all of these ideas will be highly significant in terms of impeding the intended learning.
- Some of them will. Therefore, teachers need to take learners’ ideas seriously.
The significance of learners’ ideas
Some of the ideas that students will bring to class will just appear to ‘evaporate’ away once the scientific perspective is presented. However, this is certainly not always the case. Indeed, there are a number of possible outcomes when a student (holding one set of ideas) is taught science which is inconsistent with their prior conceptions.
- Sometimes the new learning does seem to successfully supplant the old ideas without too many problems.
- Sometimes the learner treats the new ideas as if they are unrelated to their previous thinking. The science that has been learnt in school seems to be ‘stored’ separately from the existing ideas. Sometimes when this happens the student may use one set of ideas to answer formal science questions, but a different set of ideas in everyday situations
Describing learners’ ideas
Brain structure; early experience of the world; the quirks of language; things heard, seen and read out of school; and classroom experiences may all play a part in building up new ideas. All new learning is interpreted through existing ideas, so few notions that people have can be said to derive from just one source.
Two terms that are commonly used are ’alternative conceptions’ and ’alternative frameworks’.
- Alternative conception - refers to a single idea
- Alternative framework - refers to a complex or structure of related ideas.
Example of alternative conceptions
Alternative conceptions are found in all areas of science. For example, in physics, it is found that something like 85% of secondary students are likely to hold an alternative conception of the way that movement relates to force. It has been shown that students usually think that a continuously applied force will result in a body reaching a maximum speed, as an applied force gives an object a certain amount of ‘impetus’, which then somehow wears-off or dissipates.
This is one of the alternative conceptions that is known to be very tenacious, and has been found to commonly recur despite instruction in Newtonian mechanics.
An example of an alternative framework
The octet framework describes the way many students make sense of school ideas about a number of aspects of chemistry. For those who go on to study the subject at post-1 6 level, these ideas then influence how they make sense of the chemistry they are taught. Although no two students have exactly the same set of ideas, the octet framework describes aspects of student thinking that have been found to be very common.
Some aspects of the framework are quite close to scientific thinking, and others may appear quite bizarre - but it is the way these ideas can be integrated into a coherent scheme that is so significant
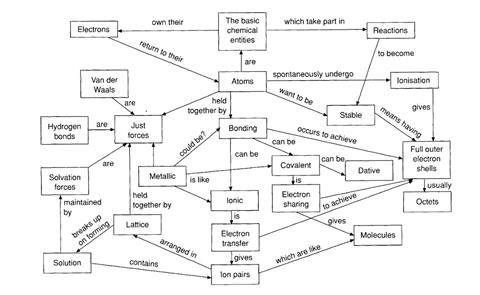
This framework appears to develop from learners’ attempts to understand why bonds form, and why reactions occur. According to this alternative framework: reactions occur between atoms (not ions, molecules, lattices etc); the reactions occur, and bonds form, so that atoms can obtain full outer shells (or octets) of electrons; and there are two ways that atoms can obtain full shells: by electron transfer (ionic bond) or electron sharing (covalent bonding).
Notes
For the full version of this chapter, see downloads below.
Downloads
Alternative conceptions in chemistry
PDF, Size 0.35 mb
Websites
Additional information
These PDFs have been taken from the popular book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 1, by Keith Taber

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
 Currently
reading
Currently
reading
Alternative conceptions in chemistry teaching
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36





























































































No comments yet