Research into learners’ ideas about these key ideas are discussed, including the types of responses that were found
Some of the classroom resources included in the companion volume were used in schools and colleges.
The chemist’s creed
This chapter is called Chemical axioms because the principles discussed here may be considered to be the basic ’tenets’ of chemistry: the key ideas upon which the whole subject is built. Learners who do not share these ‘beliefs’ - because they hold alternative conceptions -will find it very difficult to understand chemistry in the way teachers would want.
Material, energy and substance
One of the most basic discriminations in science is between energy and matter - although this is not a distinction that is always clear to students.
Although it has been realised that the division is not absolute, it is still very important at the level of secondary and college science. Energy is known to be a difficult topic for learners, and younger students may not fully appreciate that heat (for example) is not a material substance.
By the time students enter secondary school they should have overcome such problems, and recognise gases as material, and energy as something distinct from matter.
A more common problem at this level is the way that chemists tend to use the term substance (or pure substance).
Like many other words used in science, students will have heard the word ‘substance’ used with a more general everyday meaning akin to ’essence’ or ’flavour’.
To the chemistry teacher it is clear why sulfur, water and carbon dioxide are pure substances, but not wood, nor milk, nor air. Yet this distinction is based upon an appreciation of the composition of these materials at a sub-microscopic level that is not immediately available to the student.
Elements, compounds and mixtures
It is important to get learners thinking along ’chemical’ lines, and lower secondary students are usually expected to develop an appreciation of the meaning of the terms ‘element’, ’compound’ and ‘mixture’. Yet this distinction relies upon an appreciation of composition at two distinct and imperceptible levels.
A wide range of types of representation are used to show molecules in texts commonly used by students. Although this is potentially confusing for students it can also provide a context for teaching about the nature of scientific models.
The various types of diagram used to show molecules each emphasise certain aspects of the molecules, and ignore others. Students need to appreciate that molecules do not ’look’ exactly like any of these pictures, and that we select a suitable image to help explain or explore a particular aspect of the molecules.
For these particular classroom materials it was felt that modelling molecules as atomic cores in a cloud of electrons was suitable. This type of representation would enable students to clearly identify discrete molecules in the figures, and to see how many types of atomic core were present in a molecule.
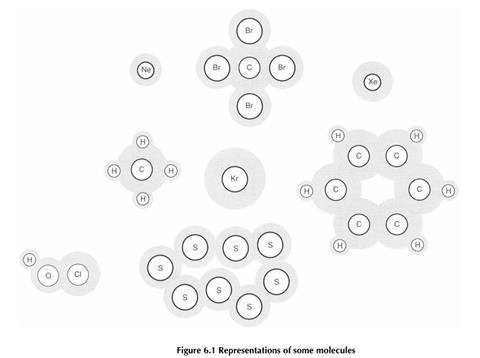
When the materials were piloted in schools it was reported by teachers that students often had difficulty explaining the key terms adequately.
Defining elements and compounds
One of the classroom activities included in the companion volume, Definitions in chemistry, is designed to explore students’ understanding of common definitions of basic chemical terms
Some of the definitions included in this activity were derived from school textbooks or common reference books.
For example, the following definition of a compound was included: ’Is made of 2 elements mixed together’.’ One student in a group of 14-1 5 year olds judged this as correct, though not helpful. This judgement was explained:
’I don’t think it is very helpful for somebody learning science because it can be made up of more than one substance’.
A classmate judged this statement as correct and helpful, ’It is correct because compounds are 2 or more elements mixed [sic] together. An element is single, they make up compounds.’
Another classmate thought the definition was wrong and unhelpful, because, ’It could be more than two elements mixed [sic] together.’
These three responses highlight just how difficult these ideas are, and the language demands they place on students. The first comment seems to suggest the student misread the definition. The second ignores the limit on two elements in the definition, and agrees that a compound is a mixture. The final student has spotted that the wording of the definition does not allow ternary (or higher) compounds, but has also agreed with the notion of a compound being a mixture.
Notes
For the full version of this chapter, see downloads below
Downloads
Chemical axioms
PDF, Size 1.17 mb
Websites
Additional information
These PDFs have been taken from the popular book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 1, by Keith Taber.

Chemical misconceptions

Discover classroom strategies and activities to tackle common misconceptions among students in chemistry, and explore the theory behind different approaches.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
 Currently
reading
Currently
reading
Chemical axioms
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36





























































































No comments yet