Gas cylinders aren’t always the best option for your learning space. This experiment will enable you to create your own, and teach students at the same time
Stand back, and watch this experiment go off with a bang, as you demonstrate to students a gas under pressure in your own learning space.
Equipment
Apparatus
- Eye protection
- 2 L plastic fizzy drink bottle (empty) with top
- Glass trough or plastic washing-up bowl
- 2-hole bung to fit bottle fitted with glass delivery tubes
- 1-hole rubber bung fitted with glass delivery tubes
- Plastic or rubber tubing with adaptors
- Spring clips
- Nichrome wire
- Side-arm flask, 250 cm3
- Delivery tube
- Tap funnel with bung to fit side-arm flask.
- Clamp stand
- Boss head
- Clamp
- Cotton thread
- 1M wooden rule
- Splint
- Sticky tape
- Balloons
Chemicals
- Hydrochloric acid, 1 mol dm-3, 250 cm3
- Magnesium turnings, 4 g
Health, safety and technical notes
- Read our standard health and safety guidance.
- Always wear eye protection.
- Hydrochloric acid is of low hazard (see CLEAPSS Hazcard HC047a).
- Magnesium turnings are highly flammable (see CLEAPSS Hazcard HC059a).
- Hydrogen is extremely flammable and explosive if mixed with oxygen. All flames should be extinguished during the preparation process.
- If the balloon is inflated with air first to stretch it, it will fill more easily with hydrogen.
Procedure
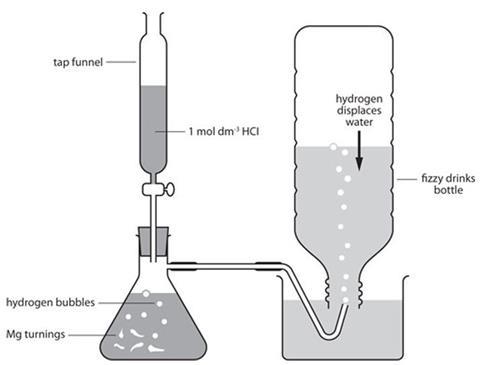
- Prepare the 2-hole bung with delivery tubes and clips as in the diagram below. Wiring up the rubber tubes with nichrome wire will prevent them being pushed off under pressure.
- Tape a splint to the 1M rule.
- Half fill the trough with water.
- Completely fill the bottle with water from the tap and screw on the top.
- Invert the bottle over the trough and remove the top. (Water will come out, and the bottle will deform at this stage. This does not matter; It will reform.)
- Place the magnesium turnings in the flask, fit the delivery tube and put it under the water.
- Fit the tap funnel and run hydrochloric acid to the flask until the level is close to the side-arm.
- Allow the reaction to run for 30 seconds at least to flush air out of the flask and delivery tube.
- Put the bottle over the delivery tube and fill it with hydrogen gas. The bottle will reform as the water is displaced with hydrogen gas.
- When all the water has been displaced, keeping the neck of the bottle underwater and fit the 2-hole bung.
- Take the bottle out of the water and stand it on its base.
- Fit a balloon to the 1-hole bung.
- Fit the other delivery tube to a laboratory water tap.
- Slowly run water into the bottle, and the balloon will inflate as the water displaces the hydrogen.
- When the balloon has been filled with hydrogen gas, tie off the balloon.
- Tie a cotton thread to the balloon and allow it to float in the air.
- Remind audience to cover their ears.
- Light the splint and ignite the balloon.

Notes
- A balloon filled with pure hydrogen will rise to the ceiling if not tied down.
- A large balloon may need 4 L of gas. (Small balloons will float with 2 L of hydrogen gas, larger ones may need more.
- More hydrogen can be forced into a balloon if more hydrogen is generated and collected in a larger container, eg a 4L milk bottle.)
- 4 dm3 of pure hydrogen in a balloon burns with a loud ‘woof’ and won’t damage ears, however some air may get into the balloon, so exercise caution.
- This method could be used to fill a balloon with a stoichiometric (exactly reacting) mix of hydrogen and oxygen. DO NOT DO THIS. The resulting explosion could have disastrous consequences of both people and property.
- Filling a balloon with oxygen gas and asking pupils what will happen when it is ignited and why often helps with misunderstandings. Many pupils predict an explosion ‘because oxygen is a reactive gas’.
- Filling balloons with equal volumes of carbon dioxide, oxygen, methane and hydrogen allows students to consider the density of gases.
Downloads
An alternative to using compressed gas cylinders - teacher notes
PDF, Size 0.21 mb
Additional information
This practical is part of our Chemistry for non-specialists collection. This experiment was written by Mike Thompson on behalf of the RSC.


















No comments yet