Use this teacher demonstration to illustrate the reactions of lithium, sodium and potassium in air and in chlorine
In this practical, students observe what happens when three group 1 metals are heated in air and in chlorine. The experiment can be used as preparation for demonstrating the reactions of Group 1 metals in water, which shows more clearly the trends in reactivity of this group.
This experiment must be done as a demonstration. If you have not attempted this experiment before, it is strongly advised that you try it before performing the demonstration in front of students.
The first step is to generate chlorine, which can be done in advance, and requires a fume cupboard. The rest of the demonstration can be done in a well-ventilated laboratory. Goggles should be worn during the chlorine generation and the demonstration. The class should also wear eye protection during the demonstration.
How long the demonstration takes depends largely on how much talking you do between each part of the experiment. To speed things up a bit, the metals can be pre-cut into appropriately sized pieces, but they should be returned to the oil until just before they are used. For some classes it may be appropriate to do just one part of the experiment and heat the metals in either air or chlorine.
Equipment
Apparatus
- Eye protection (including goggles for the demonstrator)
- Fume cupboard (only for generating the chlorine)
- Clean, dry bricks with at least one flat surface, x3 (see note 9 below)
- Gas jars with lids, x3 (see note 9)
- Bunsen burner
- Heat resistant mat
- Scalpel
- Forceps or tweezers
- Tile
- Filter paper
- Universal indicator paper
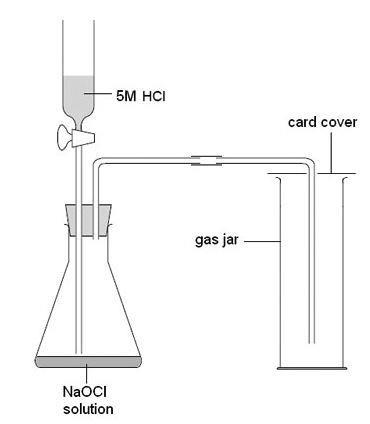
- Chlorine generator (TOXIC, DANGEROUS FOR THE ENVIRONMENT) (see note 10 below, plus the standard guidance on generating, collecting and testing gases)
Chemicals
- Lithium (HIGHLY FLAMMABLE, CORROSIVE) (see notes 11 and 12 below)
- Sodium (HIGHLY FLAMMABLE, CORROSIVE) (see note 11)
- Potassium (HIGHLY FLAMMABLE, CORROSIVE) (see note 11)
- Sodium chlorate(I) solution (also called sodium hypochlorite), 10–14% (w/v) (CORROSIVE), fresh (see note 10)
- Hydrochloric acid, 5 M (IRRITANT AT THIS DILUTION) (see note 10)
Health, safety and technical notes
- Read our standard health and safety guidance.
- The demonstrator and all students should wear eye protection throughout. The demonstrator should wear goggles or a face shield.
- Chlorine – see CLEAPSS Hazcard HC022a.
- Lithium – see CLEAPSS Hazcard HC058a.
- Sodium – see CLEAPSS Hazcard HC088.
- Potassium – see CLEAPSS Hazcard HC076.
- Sodium chlorate(I) – see CLEAPSS Hazcard HC089.
- Hydrochloric acid – see CLEAPSS Hazcard HC047a and Recipe Book RB043.
- The mouth of the gas jar must be narrower than the brick, to reduce the amount of gas escaping during the demonstration.
- There are two methods given in the standard techniques for generating chlorine. The method that uses sodium chlorate(I) is safer than the method that uses potassium manganate(VII), but will not work well if the sodium chlorate(I) is an old sample as the concentration will be too low. Note that sodium chlorate(I), NaOCl (also known as sodium hypochlorite), is NOT the same as chlorate(V), NaClO3.
- It is very helpful to have the 3 mm cubes of lithium, sodium and potassium cut ready to use. These should still be kept under oil until they are required.
- Do not heat lithium in crucibles or other porcelain material – explosions have occurred.
Procedure
Heating the metals in air
- Starting with lithium, use the tweezers to pick up a small piece of metal and place it on a tile. Use a scalpel to cut a small cube with an edge of about 3 mm. Show the students the freshly cut surface which soon tarnishes, showing that the metal reacts quickly with oxygen. Blot off the oil using the filter paper, and place it onto the flat surface of the brick.
- Heat the metal from above using the hottest part of a roaring Bunsen flame just beyond the blue cone. Once the metal is on fire, remove the Bunsen flame. You should be able to observe the classic red of a lithium flame. (You may initially see a yellow flame, but this is the burning of any oil which was not removed.) The oxide fumes from the burning metal are CORROSIVE and must NOT be inhaled. Small amounts in a well-ventilated lab are acceptable for this demonstration but large amounts must be in a fume cupboard.
- Once the metal has stopped burning, test the residue with damp indicator paper and show that it is alkaline.
- Repeat for sodium and potassium.
Heating the metals in chlorine
- Prepare gas jars on chlorine in advance, using a chlorine generator (see standard techniques for generating, collecting and testing gases).
- Check that the mouths of the gas jars of chlorine are narrower than the brick to reduce the amount of escaping gas, and that the colour of the gas in the jar is green. If it is not then there is not enough chlorine present for the demonstration to be successful.
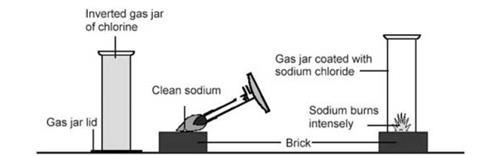
- Starting with lithium, cut a small cube with an edge of about 3 mm. Blot off any excess oil. Place it on the clean, dry brick.
- Heat the piece of metal from above using the Bunsen burner (see diagram below). When the metal is burning, take away the Bunsen, invert the gas jar, remove the lid and immediately place over the burning metal. It helps to have a second pair of hands to do this. The metal continues to burn, producing fumes of white chloride. This method avoids producing FeCl3 or CuCl2, which can occur when a combustion spoon, or deflagration spoon made of iron or brass, is used.
- Repeat for sodium and potassium.
Teaching notes
When heating in both air and chlorine, the expected pattern of lithium being the least reactive through to potassium being the most reactive may not be observed as it is hard to see potassium burning without the Bunsen flame. This may well be due to the potassium reacting faster than the others and an oxide coating being formed almost as soon as you begin to heat it.
In air:
4Li(s) + O2(g) → 2Li2O(s)
Sodium and potassium produce a mixture of oxides, peroxides and super-oxides.
In chlorine:
2Na(s) + Cl2(g) → 2NaCl(s) and similarly for the others.
The typical flame colours for lithium (red) and sodium (yellow) can usually be seen and sometimes the lilac of the potassium flame.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet