Urine luck with this practical experiment that explores colorimetry and dilution, tasking students to use their observation skills
A nineteen year old male has recently collapsed. His doctor would like the students to test;
i) the patient’s urine for glucose
ii) the concentration of salicyclic acid (the break down product from aspirin) in the patient’s urine [by colorimetry of the iron (III) salicylate complex]
iii) the patient’s blood alcohol level (by interpretation of GC’s provided)
Using this information the students are asked to make a recommendation as to the reason why the patient fainted.
Pre-lab questions
(Remember to give full references for any information beyond A-level that you find out)
- Glucose is a reducing sugar
- a) Draw the structure of glucose
- b) Use your knowledge of chemical tests in biology or chemistry to indicate a test you could use to show the presence of glucose in an unknown sample
- c) Why is the presence of glucose in a patient’s urine a cause for concern?
- Aspirin is one of the most widely used painkillers in the world today. It works by inhibiting the formation of prostaglandins which are the chemicals responsible for the sensitisation of nerve endings.
- a) Draw the structure of aspirin and highlight the functional groups that you recognise.
- b) In the digestive tract, the aspirin is hydrolysed under basic conditions to form salicyclic acid and ethanoic acid, before being absorbed into the blood stream. Write an equation for the hydrolysis reaction.
- c) The reaction in fact occurs under mildly alkaline conditions in the digestive tract. Draw the structure of salicylic acid under these conditions.
- Gas chromatography or GC is an analytical technique that can be used to identify unknown substances in a sample. Explain how GC is used to test athlete’s blood or urine for drug taking. How is the process quantitative?
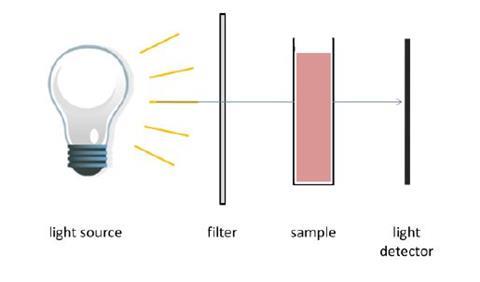
- Colorimetry is an analytical technique that can be used to determine the concentration of a coloured compound in a solution by measuring the absorbance of light by the sample relative to a sample of the same substance of known concentration. A simple diagram of a colorimeter is shown below;
- a) Explain the purpose of the filter. How is the appropriate filter for a solution chosen?
- b) Salicyclic acid can be detected in a urine sample by complexation of the salicylate anion with Fe3+ ions. A six co-ordinate complex, [Fe(salicylate)(H2O)4]+ is formed. This complex absorbs light at 520 nm and therefore appears to be pink/purple in colour.
- I. Draw the structure of the complex formed.
- II. What colour of filter should be fitted when using a colorimeter to record the absorbance of a solution of this complex?
Equipment
Each group will need a “urine” sample made of:
- 12 mg sodium salicylate [Harmful; Irritant]
- 100 mg glucose [Low hazard]
- 5 drops of ethanol [Highly flammable]
- Made up to 50 cm3 with dilute cold tea (to create a “urine” look)
For the glucose test
- Test tube
- Test tube holder
- Disposable pipettes
- Access to Benedict’s solution
- Beaker, 250 cm3
- Bunsen
- Tripod and gauze for a hot water bath
- Or Access to Clinistix®
For the colorimetry
- Access to a colorimeter
- Plastic cuvettes x 6
- 50 cm3 of a 5% by mass solution of iron(III) chloride [Irritant] (made by dissolving 4.16 g of FeCl3•6H2O in WATER† and making up to 50 cm3)
- An accurate method of measuring 1 cm3 and 4 cm3 (either 2 × 5 cm3 plastic syringe or 2 × 10 cm3 measuring cylinder)
- Disposable pipettes
- A 500 mg dm–3 stock solution of salicylate [Harmful] (made by dissolving 584 mg of sodium salicylate in 1 dm3 of water). 50 cm3 per group should be sufficient. This can be placed in a burette to facilitate the dispensing of small quantities.
- Equipment for accurate dilution of the stock solution (e.g. a burette of distilled water and small test tubes and bungs)
- Distilled water
- Test tube rack
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection.
- Wear clothing protection if desired.
- Iron(III) chloride is an irritant, see CLEAPSS Hazcard HC055b
- Salicylate is harmful, see CLEAPSS Hazcard HC052
† NOTE If the iron (III) chloride solution is made up in acid as recommended by CLEAPSS it protonates the sodium salicylate and no complex forms. It must therefore be made up IN WATER.
Extension discussion points
- Why are solutions of iron(III) ions more acidic than ethanoic acid? How does this help to explain the formation of the [Fe(H2 O)4 (salicylate)]+ complex?
- What should be used for the blank in the colorimetry experiment?
Downloads
Patient prognosis student sheet
PDF, Size 0.46 mbPatient prognosis teacher & tech sheet
PDF, Size 0.37 mb
Additional information
This resource was developed by Catherine Smith, RSC School Teacher Fellow at the University of Leicester 2011 – 2012, produced as part of the National HE STEM Programme.

Problem based practical activities
Discover how chemistry can relate to real world problems, so students can put their science knowledge into context.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
 Currently
reading
Currently
reading
Patient prognosis











































No comments yet