Help student address a thirst for knowledge with this practical
The students are set the problem of designing a new drinks container which will cool 100 cm3 of a drink by 5 °C in 5 min. The students need to decide which of ammonium nitrate and ammonium chloride should be used based on the enthalpy of solution, the solubility is in water, the cost and the relevant health and safety information for each salt. They then need to trial their method and modify the quantity of salt required accordingly.
Pre-lab questions
(Remember to give full references for any information beyond A-level that you find out)
- Give definitions for each of the following enthalpy changes. In each case include an equation to represent the process to which the enthalpy change applies;
- a) Enthalpy of solution of the ionic compound X+ Y–
- b) Lattice energy of the ionic compound X+ Y–
- c) Enthalpy of hydration of the gaseous ion Z+ (g)
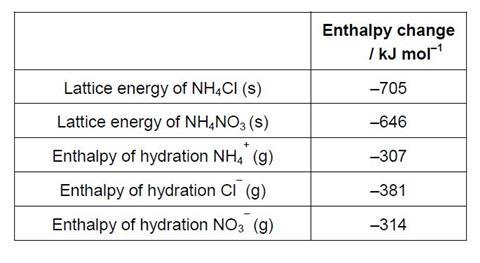
- The enthalpy of solution of an ionic compound can be calculated from its lattice enthalpy and the enthalpies of hydration of the individual ions. Use the enthalpy changes shown and a Born-Haber type cycle to calculate;
- a) The enthalpy of solution of ammonium chloride, NH4Cl (s)
- b) The enthalpy of solution of ammonium nitrate, NH4NO3 (s)
- In 2001, Nescafe launched a self-heating can of coffee. To heat up the coffee a button is pressed which mixes the heating ingredients; a single step mixes calcium oxide and water to produce calcium hydroxide and generate heat;
CaO + H2O Δ Ca(OH)2 DH –82 kJmol–1
The can warms up 210 cm3 of coffee by 40◦C.
- a) Assuming that the heat capacity for coffee is the same as that of water (4.18 JK–1g –1) calculate the energy needed to warm 210 cm3 of coffee by 40◦C.
- b) Use this value to hence calculate the minimum mass of CaO needed in the can for it to function as specified.
(Question taken from 2003 International Chemistry Olympiad Booklet 2003 – Round 1)
Equipment
Apparatus
- Accurate thermometer
- Copper calorimeter (or similar), 100 cm3
- Beaker, 250 cm3
- Measuring cylinder, 100 cm3
- Top pan balance
- Stirring rod
- Spatulas
- Insulation; Paper / aluminium foil / cotton wool etc.
- Boss, clamp and stand
- Graph paper
- Stopclock
Chemicals
- Ammonium chloride, NH4Cl [Harmful], 120 g
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection.
- Ammonium chloride is harmful, see CLEAPSS Hazcard HC047b
- Ammonium nitrate is combustible, see CLEAPSS Hazcard HC008
Ammonium nitrate requires very careful handling (R8: Contact with combustible material may cause fire, R9: Explosive when mixed with combustible material, Do not allow the salt to become contaminated with organic matter and do not grind it.)
As a result, ammonium nitrate should NOT be provided to the students under any circumstances.
More resources
Show this video of patent attorney, Charley who describes his role in making an impact by helping inventors get legal protection for their new inventions and medicines.
Downloads
Cool drinking student sheet
PDF, Size 0.23 mbCool drinking teacher & tech sheet
PDF, Size 0.44 mb
Additional information
This resource was developed by Catherine Smith, RSC School Teacher Fellow at the University of Leicester 2011 – 2012, produced as part of the National HE STEM Programme

Problem based practical activities
Discover how chemistry can relate to real world problems, so students can put their science knowledge into context.












































No comments yet