Try this microscale practical to identify and explain patterns in the solubility of fluoride, chloride, bromide and iodide anions
In this microscale experiment, students add solutions containing lithium, calcium and silver cations to solutions containing fluoride, chloride, bromide and iodide anions. They record which combinations produce an insoluble precipitate, and use their results to identify and explain patterns of behaviour among these anions.
A well-organised group of students, working in pairs, should be able to complete the practical stage in 10 minutes, given the simplicity of the procedure. Discussion of the results to identify patterns should take about the same time, but further discussion to produce explanations will depend on the level of the class.
Equipment
Apparatus
- Eye protection
- Clear acetate sheet (eg OHP sheet), or clear plastic spotting plate (well-plate) (see note 10 below)
Chemicals
- Solutions (about 2 cm3) contained in plastic pipettes (see note 11 below)
- Silver nitrate, 0.1 M
- Lithium bromide, 1 M
- Calcium nitrate, 0.4 M
- Sodium fluoride, 0.5 M (HARMFUL)
- Sodium chloride, 0.2 M
- Potassium bromide, 0.2 M
- Potassium iodide, 0.2 M
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Silver nitrate solution, AgNO3 (aq) – see CLEAPSS Hazcard HC087 and CLEAPSS Recipe Book RB077.
- Lithium bromide solution, LiBr(aq) – see CLEAPSS Hazcard HC047b.
- Calcium nitrate solution, Ca(NO3)2 (aq) – see CLEAPSS Hazcard HC019B.
- Sodium fluoride solution, NaF(aq), (HARMFUL) – see CLEAPSS Hazcard HC095C.
- Sodium chloride solution, NaCl(aq) – see CLEAPSS Hazcard HC047b and CLEAPSS Recipe Book RB082.
- Potassium bromide solution KBr(aq) – see CLEAPSS Hazcard HC047b.
- Potassium iodide solution, KI(aq) – see CLEAPSS Hazcard HC047b and CLEAPSS Recipe Book RB072.
- As an alternative to acetate sheets, transparent spotting plates (well-plates) can be used, although may be difficult to obtain. Ordinary white spotting plates should still allow precipitates to be observed, although not as easily.
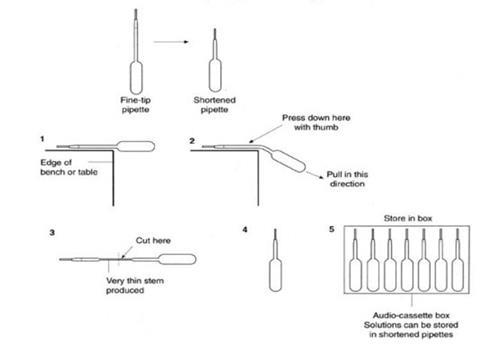
- Solutions for microscale experiments such as this can be stored, distributed, and used in specially prepared plastic pipettes. The starting point is a supply of fine-tip plastic pipettes, available in bulk from, for example, Sigma-Aldrich (cat. ref. Z13,503-8). The pipettes are adapted as shown in the diagrams below, filled with about 2 cm3 of one of the solutions, labelled, and stored as sets of seven different solutions in old audio-cassette boxes or other suitable container. Labels should include hazard information.
Procedure
- Create and duplicate a worksheet that can be covered by the acetate sheet, based on this table, scaled up to A4.
| Silver nitrate solution | Lithium bromide solution | Calcium nitrate solution |
|---|---|---|
| Fluoride ions | Fluoride ions | Fluoride ions |
| Chloride ions | Chloride ions | Chloride ions |
| Bromide ions | Bromide ions | Bromide ions |
| Iodide ions | Iodide ions | Iodide ions |
- Cover the worksheet with a clear acetate sheet.
- Put one drop of each of the halide ion solutions in the appropriate box on the acetate sheet.
- Add one drop of silver nitrate solution to each box in the first column.
- Add one drop of lithium bromide solution to each box in the second column.
- Add one drop of calcium nitrate solution to each box in the third column.
- Record all observations in a suitable table.
- Wash the acetate sheet under the tap and dry with a paper towel.
Teaching notes
This experiment looks at the similarities and differences in some of the properties of the simple halide anions. Each of the halogens forms a singly-charged anion.
Collecting students’ results should confirm:
- Silver fluoride is soluble whereas the other silver halides are insoluble.
- Calcium fluoride is insoluble while the other calcium halides are soluble.
- Lithium fluoride, although insoluble, is difficult to precipitate even at high concentrations.
Depending on their level, students should be capable of writing both formal and ionic equations for the precipitation reactions observed.
For example:
NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
and the associated ionic equation:
Ag+(aq) + Cl− (aq) → AgCl(s)
The problem with lithium fluoride, if encountered, may be due to the effect of ion pairing in solution. This is due to the high surface densities of charge on both cation and anion, so that the separate ion activities are insufficient to reach the solubility product. The other lithium halides are all very soluble.
The general conclusion is that fluorides behave rather differently from the other halides. This phenomenon is common in all groups, where the first element generally has some anomalous properties.
Follow up discussion could focus on possible reasons for this, depending on the level of the class involved. Important factors include:
- Ion size: the smaller the anion, the greater the concentration of charge on the surface of the ion, leading to stronger forces of attraction with a given cation.
- Hydration energy: the smaller an ion, the greater the attraction for the water molecules surrounding (solvating) it.
- Lattice energy: the stronger the forces between cation and anion, the more energy will be released when then come together to form a lattice; conversely, the more the energy required to prise them apart.
However there is a lot more to these phenomena than this, and teachers will wish to judge for themselves the extent and depth of the discussion that is appropriate for the students involved.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet