Try this quick teacher demonstration to demonstrate the increase in mass as iron wool is heated in air
In this experiment, students observe as iron wool is heated in air on a simple ‘see-saw’ balance. As the reaction between iron and oxygen produces iron oxide, the mass increases.
This demonstration takes around 5 minutes once it has been set up.
Plan a lesson using this demonstration
This demonstration can be used as part of a lesson plan with activities for 11–14 year olds, investigating what happens to particles during the combustion of iron – see How are particles rearranged when iron burns in air?.
Equipment
Apparatus
- Eye protection
- Bunsen burner
- Heat resistant mat
- Wooden metre rule (see note 4 below)
- Aluminium cooking foil, about 10 cm x 10 cm
- Retort stand, boss and clamp
- Plasticine, a few grams
- Knife edge, triangular block or something similar
Chemicals
- Iron or steel wool, about 4 g
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Iron or steel wool, Fe(s) – see CLEAPSS Hazcard HC055A.
- A shallow groove cut across the width of the ruler at the 50 cm mark will help when balancing it on the knife edge. Cover the end of the meter ruler with foil to protect it from the Bunsen burner.
Procedure
- Cover one end of the meter ruler with foil to protect it from the Bunsen burner. Take about 4 g of steel wool and tease it out so that the air can get around it easily. Use a few of the strands to attach it to the end of the ruler.
- Balance the ruler on a knife edge or triangular block at the 50 cm mark. Weigh the empty end with plasticine until this end is just down (see the diagram below). This part is critical.
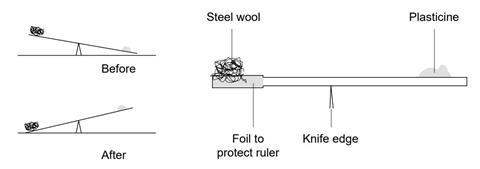
- Place a heat resistant mat underneath the steel wool.
- Wear eye protection. Light the Bunsen burner and heat the steel wool from the top with a roaring flame. It will glow and some pieces of burning wool will drop onto the heat resistant mat. Heat for about a minute by which time the meter ruler will have over-balanced so that the iron wool side is down.
Teaching notes
As you are setting up, ask the students whether they think the iron wool will go up, down or remain the same. Many will predict a weight loss.
If fine steel or iron wool is used then it may be possible to light it using a splint.
Equation:
Iron + oxygen → iron oxide
2Fe(s) + 3/2 O2(g) → Fe2O3(s)
You may also wish to look at experiment try this student experiment investigating the change in mass when magnesium burns.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet