Use this class practical to produce aspirin in a microscale esterification reaction using phosphoric acid as a catalyst
In this experiment, students prepare aspirin (2-ethanoyloxybenzenecarboxylic acid) from the reaction between salicylic acid (2-hydroxybenzoic acid) and ethanoic anhydride. The esterification reaction is catalysed by phosphoric acid, and forms white crystals.
The practical work should take around 20 minutes.
Equipment
Apparatus
- Eye protection
- Beaker, 10 cm3
- Hot plate
- Measuring cylinder, 5 cm3
- Beaker, 50 cm3
- Test tube
- Small filter funnel
Chemicals
- 2-hydroxybenzoic acid (salicylic acid)
- Ethanoic anhydride
- Phosphoric acid (85%)
Procedure
Wear splash-resistant goggles and work in a fume cupboard.
- Half-fill a 50 cm3 beaker with deionised water, and heat to 70–80 °C.
- Weigh 0.23 g of 2-hydroxybenzoic acid (salicylic acid) into a test tube.
- Add 25 drops of ethanoic anhydride followed by one drop of 85% phosphoric acid.
- Place in the water bath and leave for 15 minutes.
- While still warm add 1.5 cm3 of deionised water (use the measuring cylinder) and cool to room temperature until crystallisation begins, then cool in an ice bath.
- Filter through a small filter funnel and recrystallise in a test-tube using a mixture of 0.7 cm3 ethanol and 2 cm3 of deionised water.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout (splash-resistant goggles to BS EN166 3).
- This experiment should be done in a fume cupboard.
- 2-hydroxybenzoic acid – see CLEAPSS Hazcard HC052. 2-hydroxybenzoic acid (salicylic acid) carries the hazard warning DANGER this substance is harmful if swallowed, is suspected of damaging the unborn child, is harmful to aquatic life with long-lasting effects and may cause an allergic skin reaction.
- Ethanoic anhydride – see CLEAPSS Hazcard HC039. Ethanoic anhydride is CORROSIVE, HARMFUL if swallowed or inhaled and FLAMMABLE.
- Phosphoric acid – see CLEAPSS Hazcard HC072. Phosphoric acid (85%) is CORROSIVE.
Teaching notes
The reaction that takes place in this experiment is illustrated by the diagram below.
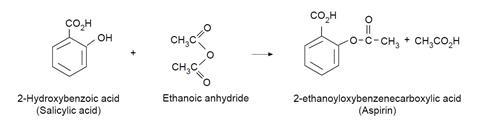
Observations
This esterification reaction, which uses reactive ethanoic anhydride and phosphoric acid catalyst, is quite fast at microscale. A good yield of white crystals should be formed.
Downloads
Microscale synthesis of aspirin - student sheet
Editable handout | Word, Size 0.1 mbMicroscale synthesis of aspirin - student sheet
Handout | PDF, Size 0.17 mbMicroscale synthesis of aspirin - teacher notes
Editable handout | Word, Size 0.1 mbMicroscale synthesis of aspirin - teacher notes
Handout | PDF, Size 0.19 mb
Additional information
This resource is part of our Microscale chemistry collection, which brings together smaller-scale experiments to engage your students and explore key chemical ideas. The resources originally appeared in the book Microscale chemistry: experiments in miniature, published by the Royal Society of Chemistry in 1998.
© Royal Society of Chemistry
Health and safety checked, 2018


















1 Reader's comment