- Home
- I am a …
- Resources
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- Literacy in science teaching
- More …
- Climate change and sustainability
- Alchemy
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Collections
- Education in Chemistry
- Teach Chemistry
- Events
- Teacher PD
- Enrichment
- Our work
- More navigation items
Structure and bonding pre-16
Classroom resources featuring activities from our Structure and bonding pre-16 professional development course for teachers
This collection is most valuable to those who have attended this course and wish to put into practice with their students some of the ideas and activities presented as part of that event. Please note that this list is not exhaustive; not all trainer activities have a corresponding classroom resource. In some circumstances there is variation between the training resource and classroom resource.
The structure and properties of chocolate
Investigate how melting chocolate changes its structure and affects properties like taste, texture and melting point. Includes kit list and safety instructions.
A solid–solid reaction between lead nitrate and potassium iodide
Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide.
Diffusion in liquids
Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Includes kit list and safety instructions.
Chemical misconceptions II: Spot the bonding | 16–18 years
This activity explores learners knowledge of different bonding types through 18 diagrams
Chemical misconceptions II: Interactions
Support students to explore more forms of interactions between bonds, identify, and explain them.
Allotropes of sulfur
Use this practical to explore the changes in the colour and consistency of sulfur as you heat it, melt it and eventually boil it. Includes kit list and safety instructions.
Heating group 1 metals in air and in chlorine
Use this demonstration to illustrate the reactions of lithium, sodium and potassium in air and in chlorine. Includes kit list, video and safety instructions.
Iron and sulfur reaction
This demonstration or class experiment shows the exothermic reaction of iron and sulphur. Includes kit list and safety instructions.
Chemical misconceptions II: Hydrogen fluoride
Explore hydrogen fluoride, discuss its chemical reaction and encourage learners to think about why the reaction takes place.
Chemical misconceptions II: Chemical stability
Explore seven triads of chemical species and understand the stability between the three examples.
Trends in reactivity in the periodic table
This could be used to follow up some work on the periodic table where the trends in reactivity in groups 1 and 7 have been identified. It can be used as a differentiated activity for the more able students within a group.
Jets of liquids: the effect of charged plastic on water
Polar covalent bonds, and their affects on water are explored in this open-ended problem.
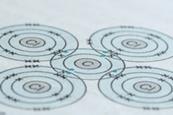
Chemical misconceptions II: An analogy for the atom
Discover more around the solar system analogy for the structure of an atom. Looking closer at their similarities and differences.
Reacting aluminium and iodine
Illustrate the spectacular reaction between aluminium and iodine with water as a catalyst in this demonstration. Includes kit list and safety instructions.
Making silicon and silanes from sand
Create silicon in your classroom using just sand and magnesium. This exothermic practical will show learners the nuances of heat based reactions and how to perform them safely. Kit list and safety instructions included.
Supercooling and the energetics of freezing
Explore what happens when a liquid is supercooled and develop learners’ observation skills in this class practical
Ionic bonding and electron transfer | Stretch and challenge | 14–16 years
Learners evaluate and discuss ideas about ionic bonding, the formation of ions and energetics
Covalent bonding
This activity seeks to develop an understanding of covalent bonding in terms of energetic stability rather than full shells.