Show learners an exothermic reaction of iron and sulfur with this demonstration and class experiment
-

Download this
A ready-to-go practical lesson with classroom slides, scaffolded and unscaffolded student worksheets and teacher guidance, including full technical notes and answers to all questions.
Discover more resources from the Nuffield practical collection
Show learners how, when chemical reactions occur, atoms are rearranged to form different substances. The original elements, once combined, have different chemical and physical properties than when they were reactants. This experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound iron sulfide. The two solids are mixed and heated in a test tube (or ignition tube). Use the reaction to illustrate elements, mixtures and compounds.
Carry out the reaction as a demonstration or class experiment in a well-ventilated laboratory, making sure learners follow instructions closely.
Carry out the reaction in borosilicate glass test tubes as a demonstration or allow learners to carry out the reaction themselves in smaller (ignition) tubes. The reaction provides an opportunity to show that the properties of a compound are different from its constituent elements.
The reaction must not be carried out on tin lids in the open laboratory as is suggested in some sources. The sulfur may boil or burn releasing sulfur dioxide which is a TOXIC and CORROSIVE gas and may trigger an asthma attack.
You can see this reaction in action in the video below.
Learning objectives
- Recall definitions of elements and compounds.
- Safely heat a mixture of two elements and record observations.
- Calculate masses using the principles of conservation of mass.
- Plot data and make predictions from the graph.
Scaffolding
There are two versions of the student worksheet: scaffolded (✪) and unscaffolded (✪✪). The scaffolded sheet offers more support to allow learners to access the questions.
For example, on the scaffolded sheet, gap-filling questions are provided for definitions. The sheet also supports learners’ understanding of the conservation of mass questions by providing annotated word equations, and for question 7, the graph paper provided has axes pre-populated with numbers and axis labels.
Technician notes
Read our standard health and safety guidance and carry out a risk assessment before running any live practical.
Equipment
- Safety glasses
- Mass balance (1 or 2 decimal places)
Teacher demonstration
For the demonstration you will need:
- Test tube made from borosilicate glass (e.g. Pyrex)
- Bunsen burner
- Heat resistant mat
- Clamp stand and clamp
- Spatulas x 2
- Small bar magnet
- Watch glass
- Filter paper (2 pieces or use 2 weighing boats)
- Mineral wool (or mineral fibre)
Class practical
For the class practical each group of learners will need:
- Prepared ignition tube
Note: fill the ignition tubes (75 mm x 10 mm test tubes) no more than one quarter full of the iron–sulfur powder mix (see first step of the demonstration procedure). Using 0.2 g of the mixture is sufficient for the effect to be seen. Place a small plug of mineral wool in the mouth of each ignition tube. After the experiment, discard the low hazard iron(II) sulfide into the refuse.
- Bunsen burner
- Heat resistant mat
- Test tube tongs
Chemicals
- Iron powder (potential IRRITANT)
- Sulfur − finely powdered roll or flowers
Safety and hazards
- Read our standard health and safety guidance.
- Wear eye protection throughout and ensure that the lab is well ventilated.
- Iron powder, Fe(s), (potential IRRITANT) – this can cause severe irritation in eyes because the iron oxidises rapidly in the saline environment – see CLEAPSS Hazcard HC055a, refer to SSERC or to your local safety advisory body. Iron powder is preferred to iron filings. If fine sulfur powder is mixed with iron filings, it is difficult to obtain a consistent mix, because the two solids can separate.
- Sulfur, S(s) – see CLEAPSS Hazcard HC096a, refer to SSERC or your local safety advisory body. Roll sulfur or flowers of sulfur should be finely powdered using a pestle and mortar.
- Sulfur dioxide, SO2(g), (TOXIC) is formed if the sulfur catches fire – see CLEAPSS Hazcard HC097, refer to SSERC or your local safety advisory body.
Demonstration
- Prepare a mixture containing iron powder and sulfur powder in the ratio 7:4 by mass. Do this by measuring out 7 g of iron powder and 4 g of finely powdered sulfur onto separate pieces of filter paper (or use weighing boats). Mix the two powders by pouring repeatedly from one piece of paper to the other until a homogeneous mixture (by appearance) is obtained.
- Note the appearance of the pure elements and the mixture. Demonstrate that iron can be separated from the mixture by physical means. Do this by wrapping the end of a small bar magnet in a paper tissue or cling film and dipping it into a teaspoon sized heap of the mixture on a watch glass. The iron will be attracted to the magnet, but the sulfur remains on the watch glass.
- Place about 2 g of the mixture into a borosilicate test tube.
- Insert a plug of mineral wool (mineral fibre) into the mouth of the test tube. Clamp the test tube as shown in the diagram.
- Heat the powder mixture at the base of the test tube – gently at first and then more strongly (use a blue flame throughout). Heat until an orange glow is seen inside the test tube. Immediately stop heating. Let the learners see that the glow continues and moves steadily through the mixture.
- Allow the test tube to cool down. At this point learners can carry out their own small-scale version of the reaction.
- Once cool, it is possible to break open the test tube to show the appearance of the product, iron(II) sulfide. Wearing protective gloves, break open the test tube using a pestle and mortar.
- It may be possible to show that the product, iron(II) sulfide is non-magnetic. However, this is not always successful. Using a very weak magnet is advisable.
Diagram
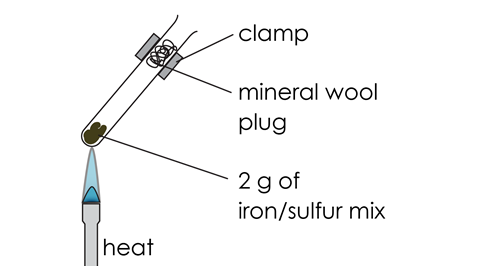
Class practical
A full method is provided in the student worksheets and duplicated here with additional information:
- You will be provided with a pre-prepared ignition tube containing the iron−sulfur mixture and a mineral wool plug.
- Using suitable tongs or test tube holders, heat the iron−sulfur mixture in the tube until it just starts to glow. Then, turn off your Bunsen burner.
- Leave the ignition tube to cool on the heat resistant mat. If possible, get learners to place all their used ignition tubes onto one heat resistant mat set aside for this purpose (e.g. on the teacher’s desk or in a fume cupboard).
Teaching notes
On heating the reaction mixture, the sulfur melts and reacts with the iron exothermically to form iron(II) sulfide. The mineral wool plug in the mouth of the test tube prevents sulfur vapour escaping and possibly catching fire. If, despite all precautions, the sulfur vapour does ignite, train learners to extinguish it by placing a damp rag firmly over the mouth of the tube.
The signs that a chemical reaction occurs are the glow and the fact that a new substance (black iron sulfide) is formed which cannot be separated by using a magnet (see step 8 of the demonstration procedure).
This is an opportunity to introduce or reinforce the rule that if only two elements are combined, the name of the compound ends in ‘ide’.
Questions
Questions linking the practical experiment to quantitative chemistry topics can be found in the student worksheets. There are two versions of the student worksheet: scaffolded (✪) and unscaffolded (✪✪). The scaffolded sheet offers more support to allow learners to access the questions. Hints are provided after some of the questions to support learners and guide their answers.
Answers
Answers to the questions in both levels of student sheets and on the lesson slides can be found in the teaching notes.
Build a lesson plan around this experiment
This experiment can be used as part of a lesson plan for 11–14 year olds, using particle models to describe the chemical change that occurs – see Reacting iron and sulfur to explore compounds.
Downloads
Iron and sulfur reaction scaffolded
Handout | PDF, Size 0.75 mbIron and sulfur reaction unscaffolded
Handout | PDF, Size 0.74 mbIron and sulfur reaction teacher
Handout | PDF, Size 0.54 mbIron and sulfur slides
Handout | PDF, Size 1.51 mbIron and sulfur reaction scaffolded
Editable handout | Word, Size 0.75 mbIron and sulfur reaction unscaffolded
Editable handout | Word, Size 0.72 mbIron and sulfur reaction teacher
Editable handout | Word, Size 0.69 mbIron and sulfur slides
Editable handout | PowerPoint, Size 3.56 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
The resource was updated in 2026 with additional follow-up questions, teacher notes and classroom slides added by Louise Glynn


















2 readers' comments