Help your students to master effective nuclear charge with these fundamental points

Our molecular world is based on charges and how these charges are regionalised over a molecule. Most post-16 students know that water and oil don’t mix, fizzy drinks go flat when left in a glass and substances exist in different states of matter at room temperature.
Indeed, immiscible liquids and solubility are fascinating and essential to understanding how cleaning products work. And inkjet printers work by directing a spray of ink using electrical charges. It is therefore important to embed learning that electrostatic charges influence states of matter, bond strength and molecular interactions.
What students need to know
Matter is a deceptively simple topic for junior pupils in terms of how we define a solid, liquid or gas. However, what we don’t mention is that charges determine the characteristics and interactions of compounds and bond strengths in the microscopic world where gravitational forces are too weak to have any effect.
Chemistry is all about charge and polarity
Put simply, chemistry is often the movement of electrons from one nucleus to another, or the attraction of opposing charges. Effective nuclear charge, the ability of a nucleus to attract (valence) electrons, is hugely important in understanding chemistry. Ionisation energy, electronegativity and intermolecular forces (and therefore states of matter, chromatography and solubility) and organic reactions mechanisms are driven by nuclear charge.
-

Download this
Bonding spectrum infographic and resources, for age range 16–18
Use this poster, fact sheet and card-based activity to help learners understand electronegativity and intermolecular forces.
Download this
Bonding spectrum infographic and resources, for age range 16–18
Improve your post-16 learners’ understanding of electronegativity and intermolecular forces with this card-based activity.
Download the poster, fact sheet and resource from the Education in Chemistry website: rsc.li/3HmTvTc
Chemistry is all about charge and polarity, which means electron location, distribution and balance. If students have a weak conceptual grasp of charge, electrostatics and polarity, this makes chemistry that bit more difficult to understand. How can we lay the foundations for better understanding?
Read all about charge and polarity for tips to teach electron location, distribution and balance in this CPD article (rsc.li/3SmjDnu). If students have a weak conceptual grasp of charge, electrostatics and polarity, this makes chemistry that bit more difficult to understand. How can we lay the foundations for better understanding?
Common misconceptions
When asked why solids are solid, students typically say that ‘the particles are close together’ and not that there are strong forces between the particles. Students often fail to understand that the forces between particles (atoms, ions, molecules) influence the state at room temperature, and that these are determined by electrostatics. By revisiting the basic concept of electrostatic forces, you can open the door to more complex understanding of molecular interactions based on polarity.
A covalent bond is an electrostatic sandwich formed by electrons being held between two positive centres
Explaining differences in the ionic radii of phosphorus (P3-) and aluminium (Al3+) can be tricky, but if students understand charge on the nucleus and the attraction it has for the electrons then these questions are more straightforward. Curly arrows depicting electron movement in (organic) reaction mechanisms can also be confusing, but it is often simply electrostatic attraction that determines electron movement.
Ideas for your classroom
Although ionic bonding is a relatively easy fit in terms of opposite ion electrostatic attraction (use static electricity to demonstrate attraction and repulsion of charges), covalent bonding is conceptually more difficult. A covalent bond is an electrostatic sandwich formed by electrons being held between two positive centres (nuclei).
The data in the table shows that increasing nuclear charge, for the same energy level across a period, means electronegativity increases and correlates with decreasing covalent radius. Shorter bond lengths correlate directly with increased bond strength. When reading down a group, covalent radius increases as shells are added to the atom and the effect of the nucleus on the outer electrons — the effective nuclear charge — decreases.
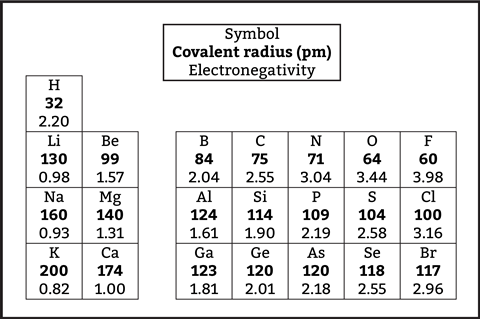
The data in the table online (rsc.li/3HmTvTc) and available on each element’s atomic data on the RSC periodic table (rsc.li/3S6fT82) shows that increasing nuclear charge, for the same energy level across a period, means electronegativity increases and correlates with decreasing covalent radius. Shorter bond lengths correlate directly with increased bond strength. When reading down a group, covalent radius increases as shells are added to the atom and the effect of the nucleus on the outer electrons — the effective nuclear charge — decreases.
Covalent molecules are a collection of positive nuclei surrounded by negatively charged electrons. Thus, all simple covalent molecules should repel each other and be gases. So, how are some of them liquids (bromine, hexane, water) or solids (iodine, fats, sugar) at room temperature? Non-polar particles, such as diatomic elements or monatomic gases, have weak intermolecular forces based on a momentarily uneven distribution of electrons.
However, polar molecules, which have a larger electronegativity difference, have a permanently uneven electron distribution that leads to permanent partial charges within the molecule. A polar molecule will have stronger intermolecular forces as a result of permanent dipole–permanent dipole interactions relative to a non-polar molecule of similar mass.
Demonstrating London dispersion forces
Have you ever stuck a balloon to the wall by rubbing it on your head? Although this phenomenon doesn’t, strictly speaking, show London dispersion forces (LDF), it’s an example of inducing a dipole from a momentarily charged substance. A more particle-relevant demonstration of the short-range attractive forces is to put a charged balloon close to an empty aluminium drinks can – the can will roll towards the balloon because of an induced dipole.
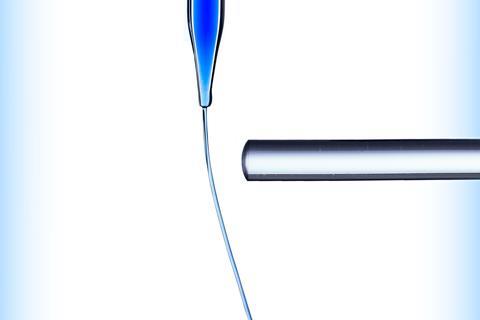
A demonstration of LDF in a covalent molecule is to allow water to flow through a burette and place a charged plastic rod next to it. The liquid jet deflects because of an induced dipole in the water from the charged rod.
Have you ever stuck a balloon to the wall by rubbing it on your head or jumper? Demonstrate and use this PhET simulation (bit.ly/3tSOsqp) to show the charges. Although this phenomenon doesn’t, strictly speaking, show London dispersion forces (LDF), it’s an example of inducing a dipole from a momentarily charged substance. A more particle-relevant demonstration of the short-range attractive forces is to put a charged balloon close to an empty aluminium drinks can – the can will roll towards the balloon because of an induced dipole.
Show LDF in a covalent molecule with this simple activity (rsc.li/3tUFJEa). Allow water to flow through a burette and place a charged plastic rod next to it. The liquid jet deflects because of an induced dipole in the water from the charged rod.
Checking for understanding
When you are teaching periodicity, such as atomic size, ionisation energy and covalent bond strength (or length), make sure you ask your students which factors are causing these effects and relate it back to effective nuclear charge.
Remind students that the increasing nuclear charges across a period lead to decreasing atomic sizes. And, that the increasing number of electron shells down a group mean that, despite increasing nuclear charge, size increases because each outer electron experiences less attraction from the nucleus.
More resources
- Use the contexts, ideas and activities to help you teach post-16 students the orbital model of the atom and bonding with this CPD article.
- Watch Katie’s video job profile. She is an executive editor and uses her chemistry background to work with scientists around the world to publish the latest research in scientific journals.
- Display the Electron configurations poster in your classroom and download the peer-assessment activity to help your post-16 learners understand orbitals and shells.
More resources
- Use the contexts, ideas and activities to help you teach post-16 students the orbital model of the atom and bonding with this CPD article: rsc.li/3Sl4UsF
- Introduce science-related careers with a video job profile of an executive editor for a scientific journal: rsc.li/3U215Ke
- Display the Electron configurations poster in your classroom and download the peer-assessment activity to help your post-16 learners understand orbitals and shells: rsc.li/47DzDWn
Check your students’ understanding by asking why heterolytic bond fission is occurring rather than homolytic, or why electrons are being ‘pushed’ in the direction they are by curly arrows in a reaction mechanism.
Take-home points
Understanding electrostatic forces is important, and with practice students can have a firm grasp of the concepts. Ensure your pupils understand that states of matter and chemical reactions are governed by electrostatic forces. Solids are solid because of strong electrostatic forces. In terms of electrostatics, the nucleus of an atom is very much overlooked in chemistry because we get distracted by the movement and ubiquity of electrons. Remember that effective nuclear charge dictates how tightly electrons are bound to that nucleus and how easily they can be removed, or repositioned.
Across a period, increasing nuclear charge decreases atomic size and increases ionisation energy. Down a group, electron shells are added and the atomic radius increases, which decreases the effect of the nuclear charge on the outer electrons. Polarity within molecules is dictated by the electronegativity of different atoms, which is governed by effective nuclear charge.
Take-home points
Understanding electrostatic forces is important, and with practice students can have a firm grasp of the concepts.
- Ensure your pupils understand that states of matter and chemical reactions are governed by electrostatic forces. Solids are solid because of strong electrostatic forces.
- Remember that effective nuclear charge dictates how tightly electrons are bound to the nucleus and how easily they can be removed, or repositioned.
- Across a period, increasing nuclear charge decreases atomic size and increases ionisation energy. Down a group, electron shells are added and the atomic radius increases, which decreases the effect of the nuclear charge on the outer electrons.
- Polarity within molecules is dictated by the electronegativity of different atoms, which is governed by effective nuclear charge.
Article written by Duncan Short, a chemistry teacher in Falkirk. Resource by Martin Bluemel, a retired head of chemistry














3 readers' comments