Point your students in the direction of these ideas and exercises when teaching reaction mechanisms
Students aged 11–14 see chemical processes as the rearrangement of elements. At 14–16, they start to see chemical processes as the rearrangement of atoms. However, post-16 students need to change their perspective to see these processes as the rearrangement of electrons.
For organic mechanisms, we use curly arrows to show the stepwise rearrangement of electrons in a reaction. Understanding mechanisms allows synthetic organic chemists to optimise reactions, for example to get predominantly the E or Z isomer around a double bond.
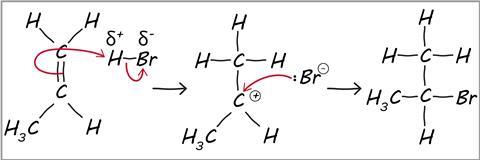
What students need to know
Hidden underneath the list of reagents, reaction conditions and things to recall, organic chemistry can be understood by applying a few fundamental principles about how electrons rearrange. For many reactions that students encounter, the reactant either accepts a new dative bond or makes one.
- Reactions are described in terms of electron movement. The movement of pairs of electrons are shown using curly arrows.
- Nucleophiles form a new (dative) bond to which they contribute both electrons. Nucleophiles mostly have a lone pair; some are negatively charged. Examples of nucleophiles are H2O, OH–, NH3, ROH and CN–. The curly arrow starts from the nucleophile.
- Electrophiles can accept a pair of electrons in the form of a new dative bond. Curly arrows go to electrophiles, which are sometimes positively charged or have a weak bond, like Br2, HCl, H+ and NO2+.
- Free radicals have an unpaired electron that tends to be quite reactive and contribute a single electron to a new bond, like Br∙, Cl∙ and ∙O∙.
Get more resources
- Get more tips for drawing curly arrows for mechanisms.
- See this teaching organic mechanisms article for some simple approaches to help students understand this topic.
- Use this online organic chemistry module for tools and tips for teaching electron pushing.
- Learn about the history of curly arrows.
Threshold concept
To understand an organic reaction and predict the products and possible side reactions, we need to understand the stepwise rearrangement of electrons and the electronic structure of any intermediates.
Where students go wrong
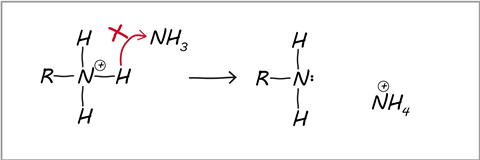
Students often apply the wrong kind of thinking to organic mechanisms, trying to recall curly arrow mechanisms rather than analyse, understand and apply the concepts of electron movement. Some recall is involved, because students need to know whether reagents are nucleophiles or electrophiles, and if they can recall the reaction product then that makes writing the mechanism easier.
Common misconceptions
Crucially, students tend to take the representations of molecules and ions at face value rather than imagining the implicit information about shape and electronic structure. By not thinking in terms of electrons, they often show arrows representing the movement of atoms or molecules and are not precise with the start and end points of their arrows. Students can struggle to correctly identify when formal charges are produced in intermediates and often overemphasise the importance of dipoles and underestimate the importance of weak bonds.
Ideas for the classroom
Increase the awareness of electrons and, in particular, counting electrons, by frequently revisiting dot and cross diagrams in the activating prior knowledge part of the lesson.
Increase the awareness of electrons and, in particular, counting electrons, by frequently revisiting dot and cross diagrams in the activating prior knowledge part of the lesson.
- Focus on ions like OH–, NH4+ and CN– and reagents such as NH3.
- Revisit molecular shapes to raise awareness of lone pairs.
- Ask students to show all the implicit electrons in the structures of reactants.
- Show free radical substitution mechanisms through fully drawn dot and cross diagrams (see diagram).
- Emphasise how many electrons from the reagent contribute to the new bond.
There is great benefit derived from dual coding – putting curly arrows into words. Encourage students to talk about where the pair of electrons started and where they ended up. Avoid language like ‘the nucleophile attacks’ and instead use ‘the pair of electrons that started as a lone pair on the oxygen forms a new dative bond to the carbon’.
Think aloud as you construct the result of a sequence of curly arrow steps. Point out to your students that only the changes shown by curly arrows can change in that step.
- Start with the carbon at the heart of the action in the reactant.
- Add each bond to it step-by-step and say if the bond is the same or has changed.
- Check the answer by using the conservation of charge check – is the total charge at the end of this step the same as the total charge at the beginning.
Use wrong mechanisms and ask the class to identify the errors and provide correct answers. This increases their ability to check to see if their mechanisms are correct.
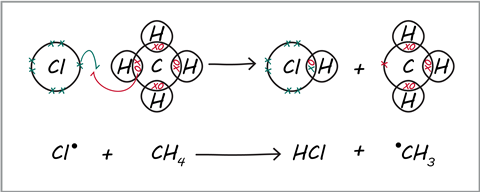
Think aloud as you construct the result of a sequence of curly arrow steps. Point out to your students that only the changes shown by curly arrows can change in that step.
- Start with the carbon at the heart of the action in the reactant.
- Add each bond to it step-by-step and say if the bond is the same or has changed.
- Check the answer by using the conservation of charge check – is the total charge at the end of this step the same as the total charge at the beginning.
Use wrong mechanisms and ask the class to identify the errors and provide correct answers. This increases their ability to check to see if their mechanisms are correct.

Get more resources
- Help your students draw curly arrows when faced with unfamiliar reactions in exams: rsc.li/3wUrEVP
- Use this article on teaching organic mechanisms to help your students get to grips with this tricky topic: rsc.li/3wXDb6I
- Ensure your students understand electron pushing with these tools and tips: rsc.li/3LXy1fu
- Walk your students through the history of curly arrows: bit.ly/3M06JoP
Assessment
It can be revealing to ask for mechanisms from past topics alongside a current topic; if students try to simply recall mechanisms, they will find this very challenging because this information will be abstract and not rely on understanding underlying concepts.
Consider using this resource to identify student misconceptions about mechanisms, or this interactive online guide to post-16 chemistry mechanisms.
Take-home points
Students need to be able to see the implicit information and realise that mechanisms must be understood rather than memorised. Learning to produce accurate mechanisms is a task that extends through most of the student’s course, but the time invested will be well rewarded. The ideas outlined in this article are designed to improve students’ understanding and their use of higher-order thinking skills.
Ready for more tips on teaching curly arrows? Read End curly arrow anxiety and download the worksheets.
Consider using resources to identify student misconceptions about mechanisms, such as worksheets (rsc.li/3NGpCOG) or interactive online guides (rsc.li/38VjdjX).
Take-home points
Students need to be able to see the implicit information and realise that mechanisms must be understood rather than memorised. Learning to produce accurate mechanisms is a task that extends through most of the student’s course, but the time invested will be well rewarded. The ideas outlined in this article are designed to improve students’ understanding and their use of higher-order thinking skills.
Tim Jolliff is an independent science education consultant and teacher of 11–18 chemistry students














2 readers' comments