Focus on real-life applications to help students get to grips with the fundamentals of this analytical technique
In the early 20th century, Mikhail Tsvet invented column chromatography. Since then, scientists have developed many more chromatographic techniques including paper chromatography, thin layer chromatography (TLC), gas chromatography and liquid chromatography.
Chemists can pair many of these techniques with tools to identify the separated components and determine their concentrations. For example, coupling chromatography with mass spectrometry, ultraviolet (UV) spectroscopy, infrared (IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy.

Download this
Chromatography investigation and questions, for age range 16–18
Explore chromatography and its applications. Plus, task learners to research how chemists use other analytical techniques to identify a mixture’s components.
Resources include:
Download this
Chromatography investigation and questions, for age range 16–18
Explore chromatography and its applications. Plus, task learners to research how chemists use other analytical techniques to identify a mixture’s components.
Download the resource from the Education in Chemistry website: rsc.li/3P9uFu6
Chromatography has myriad applications. We use it to look for drugs in blood, to test food for illegal compounds and to monitor pesticide levels, nanoplastics and heavy metals in the environment. Forensic science owes much to chromatography. Scientists also use chromatography to isolate and identify intermediates in multistage chemical reactions, to separate chiral molecules from racemic mixtures, to purify vaccines and to help search for life on Mars.
What students need to know
In chromatography, we dissolve the analyte (or mixture) in a mobile phase which is forced through a stationary phase, sometimes with the help of an inert carrier liquid or gas.
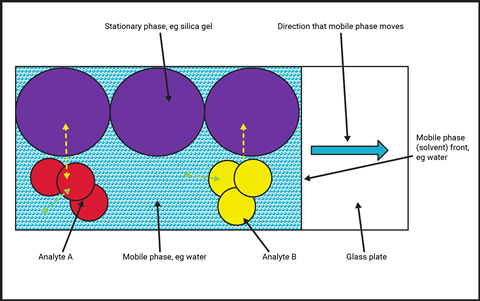
The effective separation of the components in an analyte requires a difference in strength of the intermolecular forces between each component and the stationary and/or mobile phases (Figure 1). The selection of stationary and mobile phases is critical to ensure effective separation. Components that have stronger intermolecular interactions with the stationary phase will adhere more strongly to it, and therefore move more slowly through it. Components that have weaker interactions with the stationary phase, or stronger ones with the mobile phase, will move faster. The stationary phase can be solid or liquid, whereas the mobile phase is either a liquid or gas.
The effective separation of the components in an analyte requires a difference in strength of the intermolecular forces between each component and the stationary and/or mobile phases (Figure 1). The selection of stationary and mobile phases is critical to ensure effective separation. Components that have stronger intermolecular interactions with the stationary phase will adhere more strongly to it, and therefore move more slowly through it. Components that have weaker interactions with the stationary phase, or stronger ones with the mobile phase, will move faster.
The stationary phase can be solid or liquid, whereas the mobile phase is either a liquid or gas (see table).
| Chromatography technique | Examples of use | Stationary phase examples | Mobile phase examples |
|---|---|---|---|
|
Paper |
Inks and dyes |
Cellulose – can be of different thicknesses and composition |
Water, ethanol, pentane, propanone or a mixture of these solvents |
|
Thin layer |
Steroids, amino acids, lipids, hydrocarbons, carbohydrates, amines, pesticides and vitamins |
Silica or aluminium oxide on an inert surface like glass or plastic |
Hexane and ethyl ethanoate mixtures, methanol and dichloromethane mixtures, and butanol and ethanoic acid mixtures |
|
Column |
Purines and sugars. To purify drugs and vaccines, for forensics and for food testing |
Silica, cellulose, aluminium oxide and magnesium oxide |
Hexane, ethyl ethanoate, propane, ethanol, methanol, hydrocarbons, dichloromethane or mixtures of these solvents |
|
Gas |
To monitor air quality to detect pollutants. To analyse additives and detect food contaminants |
Silica, silicone and polymers such as dimethyl polysiloxane |
Helium, nitrogen, argon and hydrogen |
Common misconceptions
Students’ most common misconceptions about chromatography include thinking they need to cover the analyte with the mobile phase when doing paper chromatography and TLC, rather than allowing the mobile phase to travel through the analytes from below the baseline. Some learners also believe chromatography has not worked on a pigment if it doesn’t move (when, in fact, that pigment is insoluble in the mobile phase), even though other pigments in the analyte have been separated.
Some students mistakenly believe the pigments move as the mobile phase pushes them along, ie it exerts a force on the pigment. Others only consider the affinity between the mobile phase and component as the reason for separation, rather than the relative interactions between the component and both the stationary and mobile phases.
Ideas for your classroom
Use the Practical chromatography resource to help students better understand the ideas behind chromatography, including solubility and Rf values. The article also describes some interesting investigations.
Download the practical chromatography resource from our website (rsc.li/3V4fFkX) to help students better understand the ideas behind chromatography, including solubility and Rf values. The article also describes some interesting investigations.
Separating mixtures of dyes is a simple but effective way to introduce the power of chromatography. For example, mix one of two different blue dyes with a yellow dye to create a green dye and ask students to identify which blue dye was used in the mixture. They should use chromatography, either TLC or paper, to analyse the green mixture and both blue dyes. This is a GCSE required practical activity you can use to remind students about the application of Rf values.
More resources
- Highlight what analytical chemists do and the different sectors they work in, such as food and pharmaceuticals.
- Get hands on with TLC to investigate the photosynthetic pigments of plants or to analyse paracetamol.
- Show your students how chemists at the National Gallery in London use chromatography to restore art works.
- Use the Structure determination question and answer sheet to test learners’ knowledge of chromatography, spectrometry and spectroscopy.
More resources
- Highlight what analytical chemists do (rsc.li/3sq7ecb) and the different sectors they work in, such as food and pharmaceuticals: rsc.li/3T95k4a
- Get hands on with TLC to investigate the photosynthetic pigments of plants (bit.ly/3IplSR9) or to analyse paracetamol in your post-16 lessons: rsc.li/3wQVtZH
- Show your students how chemists at the National Gallery in London use chromatography to restore art works: rsc.li/48K4NvX
- Use the structure determination question and answer sheet to test learners’ knowledge of chromatography, spectrometry and spectroscopy: rsc.li/438MteT
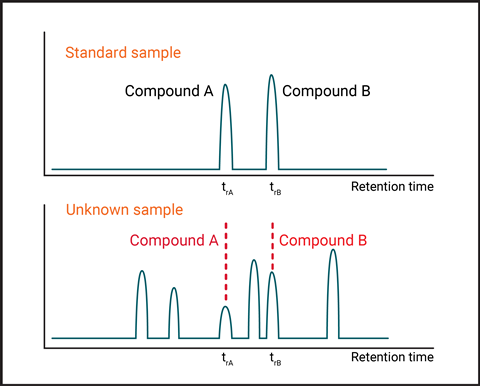
When doing gas chromatography, get your students to identify the components in a mixture using retention times – often done by comparing retention times with known compounds (Figure 2). Students can identify components A and B by using a standard containing these compounds for comparison. Taking measurements of retention times (time taken for the compound to be seen on the chromatograph, trA and trB) will help them identify the components.
Task your students to investigate food dyes to help cement chromatography. Anthocyanins – naturally occurring dyes in plants such as blueberry, blackberry, red cabbage and beetroot – often change colour depending on pH. Students can compare chromatograms of anthocyanins using neutral (water), slightly acidic (added citric acid) and alkaline (solution of NaHCO3 in water) mobile phases. Ask them how chemists could use these methods to enhance the identification of anthocyanins in food dyes?.
Students can also look for the presence of paprika extract (oleoresin) and turmeric (curcumin) in food dyes. These compounds are insoluble in water but will dissolve in oil. Students can use TLC or paper chromatography to hunt for these in food dyes, using a mobile phase of 1:1 mixture of isopropanol and water.
You can use hybrid analyses where chromatography is combined with mass spectrometry, or UV, IR or NMR spectroscopy to give learners a deeper understanding of chromatography applications.
Checking for understanding
Assess students’ knowledge of the different chromatography techniques and applications with recall questions. Understanding is best assessed through application, like the interpretation of a chromatogram.
Take-home points
- Chromatography includes a range of techniques, each suited to different purposes.
- Often, chromatography is coupled with other analytical techniques such as mass spectrometry, or IR, UV and NMR spectroscopy to enhance the information collected about the components.
- The separation of components in a mixture is not only influenced by the mobile phase but is also related to the partition between the mobile phase and stationary phase. This, in turn, is linked to the different strengths of intermolecular forces between the component, mobile and stationary phases.
- Chromatography has revolutionised forensic science and the ability to convict perpetrators of major crimes.
Article and resource by Andy Markwick, a teacher educator and research supervisor













No comments yet