Ideas and activities to help your students understand the role of catalysts and the Arrhenius equation
Whether they’re natural or synthetic, catalysts are everywhere. They’re essential for life and are used in numerous manufacturing processes to increase production rates and cut costs. Students might be familiar with the use of catalysts in the production of ammonia and sulfuric acid, but do they realise that chemists are also developing a range of catalysts to help clean pollutants in our environment?
Catalysts may also play a part in answering that famous big question: how did life begin? Some scientists have proposed that the answer lies with common mud, or more accurately the clay minerals in mud, which can act as catalysts.
What students need to know
To understand the role that catalysts play, students need a good understanding of:
- Activation energy and the interpretation of enthalpy profile diagrams and Maxwell–Boltzmann distribution curves
- The rate equation: rate = k[A]m [B]n, where m and n are the orders of reaction for reactants A and B, respectively, and k is the rate constant
- The Arrhenius equation, which shows the link between the rate constant, activation energy and temperature: k = Ae-Ea/RT, where A is the Arrhenius constant, Ea is the activation energy and T is the temperature in kelvin
- How to manipulate and interpret the Arrhenius equation with experimental data to show how changes in temperature and the addition of catalysts affect the activation energy and rate constant: lnk = lnA - Ea/RT
Threshold concept
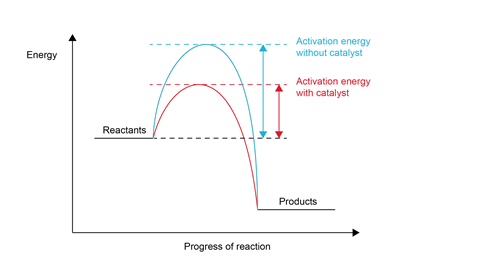
A key concept is the idea that catalysts provide an alternative reaction pathway with a lower activation energy. They do this by producing an alternative transition state or intermediate to that in the catalyst-free reaction. This may be due to adsorption of molecules onto a catalyst’s surface or active site, or a redox reaction involving different oxidation states. Either process results in a lowering of activation energies.
Misconceptions
You may find that some of your students hold one or more of these common misconceptions.
- Catalysts break chemical bonds. They don’t. A catalyst works by providing an alternative reaction pathway, with a lower energy of activation (Ea).
- Catalysts increase the yield of the product. Looking at how catalytic converters in cars work is useful here, and provides a suitable topic for further discussion.
- In a reversible reaction at equilibrium, catalysts only affect the rate of the forward reaction. If this was true, the position of the equilibrium would also be affected. Remind your students that, at equilibrium, the rates of the forward and reverse reactions are the same. Try out some of these activities.
- Catalysts ‘are not involved’ in chemical reactions. This misconception arises because many catalysts can be recovered, chemically unchanged, at the end of a reaction. The catalytic oxidation of potassium sodium tartrate (aka traffic lights) can be used to illustrate that catalysts are involved in reactions – the solution reverts from green back to the original pink colour. At pre-16 level, students are told that the mass of MnO2 before and after catalytic breakdown of H2O2 is the same.
Ideas for your teaching
When it comes to the big questions, catalysts may have some of the answers. Use these exciting contexts to get your students hooked.
Mud and life on Earth
One of science’s biggest questions is ‘how did life on Earth begin?’ Well, the answer might involve clay – a major constituent of mud – catalysing the synthesis of the precursor molecules to life. This idea, first suggested in the 1920s, is now backed up with modern experimental data.
A clay’s porous structure gives it a massive surface area, covered in potentially catalytical active sites. There’s plenty of room for lots of organic molecules to find one of these active sites and undergo a reaction, yielding key biogenic molecules such as amino acids, polypeptides and even RNA. What’s more, clay’s sponge-like structure protects reagents and products from degradation from UV light – a big factor in Earth’s early history.
Can catalysts help clean up our planet?
Plastic waste can now be efficiently converted into methane using a ruthenium-based catalyst. This new technology could help mitigate the planet’s growing plastic-waste problem while simultaneously producing methane, for use as a fuel or chemical feedstock, in a more environmentally friendly way than fracking. Introduce this topic with this starter slide and questions.
Researchers have found and developed an efficient, inexpensive new catalyst for turning atmospheric carbon dioxide into jet fuel. Such a net-zero fuel could be a major breakthrough in the fight against climate change. Use the questions and summary slide to kick start this topic with your class.
Students can also be introduced to the types of careers that employ catalysis. A wide range of examples can be found on the STEM Learning website and you can also find plenty of job profiles at A future in chemistry.

Get practical
It’s always good to use practical activities to re-enforce student ideas. Why not check out the RSC’s practical videos or have a go at the catalysis of a sodium thiosulfate and iron(III) nitrate reaction with your students.
Linking the pieces of the Arrhenius equation
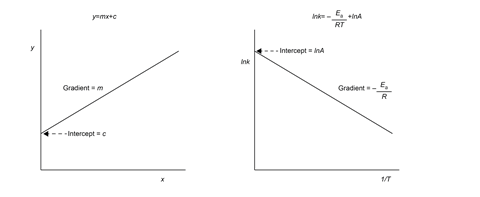
Use graphs to help your students make the links between the rate constant, reaction temperature and activation energy. For example, the natural log form of the Arrhenius equation equates to the equation of a straight line. Remind students of the simple algebraic equation y = mx + c before presenting them with the Arrhenius equation, in the natural log format. Draw students’ attention to the negative gradient of the line, telling them that’s why it slopes in the other direction compared to the example y = mx + c line. If you used the graphical representations from the checklist in Teaching rates of reaction post-16, you might find the steps described below useful; you can download them too.
Introducing the rate equation
Students should be familiar with transforming a relationship that shows a proportional relationship into an equation by including a constant of proportionality.
Rate = [x]n becomes Rate = k[x]n
Where k is the rate constant. This is only a constant when the temperature remains the same or when a catalyst doesn’t affect the rate.
Calculating the rate constant
Now take Log10
Log rate = logk + nlog[x]
This equation form can be compared to y = mx + c, where Log rate equates to y, logk to c and nlog[x] to mx.
So, plotting log[x] against log rate allows the gradient n (order) to be calculated. The intercept is logk which is important for calculating the Ea.
Calculating the activation energy (Ea)
Applying the Arrhenius equation
k = Ae-Ea/RT
Take the natural logarithms and plot lnk versus 1/T
Download this
Download the Next steps document, the Assessment question and answer sheets from the Education in Chemistry website: rsc.li/3yXw0Ki
When it comes to the big questions, catalysts may have some of the answers. And these are great contexts to hook your students into the topic.
You could start with one of science’s biggest questions is ‘how did life on Earth begin?’ Well, the answer might involve clay – a major constituent of mud – catalysing the synthesis of the precursor molecules to life. This idea, first suggested in the 1920s, is now backed up with modern experimental data.
A clay’s porous structure gives it a massive surface area, covered in potentially catalytical active sites. There’s plenty of room for lots of organic molecules to find one of these active sites and undergo a reaction, yielding key biogenic molecules such as amino acids, polypeptides and even RNA. What’s more, clay’s sponge-like structure protects reagents and products from degradation from UV light – a big factor in Earth’s early history.
Catalysts are also playing an important role in solving some of the big questions. For example, plastic waste can be efficiently converted intomethane using a ruthenium-based catalyst. This new technology could help mitigate the planet’s growing plastic-waste problem while simultaneously producing methane, for use as a fuel or chemical feedstock, in a more environmentally friendly way than fracking. Introduce this topic with this starter slide and questions (rsc.li/3COSrUG).
Alternatively, use this starter slide to discuss another efficient new catalyst, this time for turning atmospheric carbon dioxide into jet fuel (rsc.li/3slGfWr). Such a net-zero fuel could be a major breakthrough in the fight against climate change.
Catalysis is also an opportunity to introduce students to different careers. You’ll find plenty of examples on the STEM Learning website and on the Royal Society of Chemistry’s A future in chemistry.
The Arrhenius equation has a place here too. Picking up from last issue’s article (rsc.li/3yLp1nU), you can use graphs to help your students make the links between the rate constant, reaction temperature and activation energy. For example, the natural log form of the Arrhenius equation equates to the equation of a straight line. Remind students of the simple algebraic equation y = mx + c before presenting them with the Arrhenius equation, in the natural log format. Draw students’ attention to the negative gradient of the line, telling them that’s why it slopes in the other direction compared to the example y=mx+c line. You can download the Next steps document for ideas on how to do this.
Include practical activities too. They can reinforce student ideas. You could use a practical video (rsc.li/3xKkqAM) or have a go at the catalysis of a sodium thiosulfate and iron(III) nitrate reaction with your students (rsc.li/3yMwsei).
Assessment
A more creative way to assess whether students understand a concept is for them to apply their knowledge to solve a problem or to interpret and explain something. Here are four ideas to get you started. You can download these as a student question sheet and get an answer sheet too (above).
1. Show students the video of Rochelle’s salt reaction. Can they explain what evidence suggests that the reaction is catalysed?
1. Show students the video of Rochelle’s salt reaction (bit.ly/37Rgsfq). Can they explain what evidence suggests that the reaction is catalysed?
2. Ask students to describe and then explain what this diagram depicts.
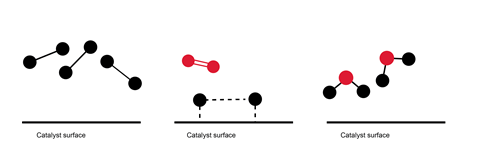
3. Present your students with these equations. Ask them to identify what represents the catalysts and what represents a transitional or intermediate state. What are the products? Can students write the overall equation for the reaction?
A + B → C + D
D + E → B + F
4. Now challenge students with this reaction equation. Can they identify the intermediate and catalyst species? Can they explain their choices?
H2O2 + I- → H2O + IO-
IO- + H2O2 → H2O + O2 + I-
Take-home points
- When teaching rates of reaction, be creative. Use up-to-date contexts and, where possible, include relevant careers to capture student interest. Catalysts are, and will remain, at the cutting edge of science and technology. Chemistry World is a great source of information.
- Be aware of common misconceptions associated with this topic and use questioning to identify them in your students. Challenge these ideas through a series of classroom activities.
- When it comes to assessment, challenge students to solve a problem or interpret and explain a reaction.
Downloads
Teaching rates of reaction post-16 Next steps
Editable handout | Word, Size 0.11 mbTeaching rates of reaction post-16 Assessment student sheet
Editable handout | Word, Size 0.13 mbTeaching rates of reaction post-16 Assessment answer sheet
Editable handout | Word, Size 0.13 mbTeaching rates of reaction post-16 Next steps
Handout | PDF, Size 0.18 mbTeaching rates of reaction post-16 Assessment student sheet
Handout | PDF, Size 0.16 mbTeaching rates of reaction post-16 Assessment answer sheet
Handout | PDF, Size 0.17 mb



















1 Reader's comment